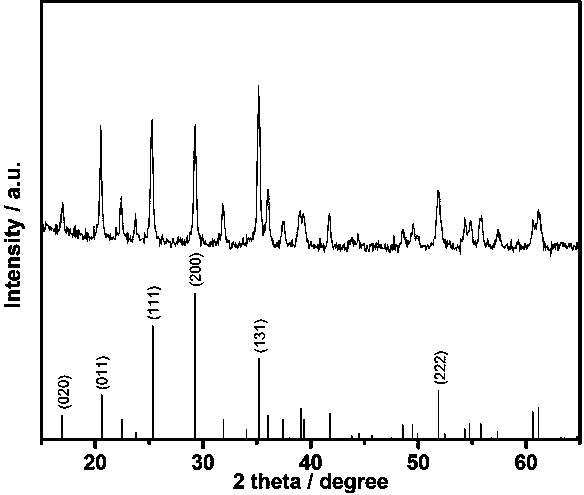
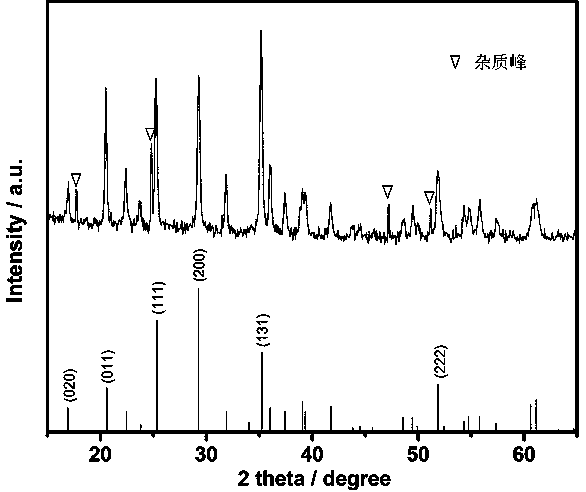
Method for preparing nano lithium manganese phosphate/graphene composite
A technology of lithium manganese phosphate and composite material, which is applied in the field of preparation of nanometer lithium manganese phosphate/graphene composite material, can solve the problems of high cost and complicated operation, and achieve the effects of low cost, simple operation and simple process operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Dissolve 6g of glucose in 60mL of ethylene glycol and 2 Insulate at 140°C for 2 hours under protection, so that the color of the ethylene glycol solution changes from colorless to light yellow, which indicates that ethylene glycol glucoside surfactants are generated in the ethylene glycol solution, and a light yellow solution A is finally obtained. 31.4 mg of graphene oxide was ultrasonically dispersed into solution A to obtain solution A containing graphene oxide. Take 0.06mol lithium hydroxide (LiOH·H 2 O) Dissolve in 15mL deionized water, mix it with solution A and stir evenly to obtain solution B. Take 0.02mol manganese sulfate (MnSO 4 ) and 0.02mol phosphoric acid (H 3 PO 4 ) was dissolved in 15mL deionized water to obtain solution C, and solution C was added to solution B to form a reaction solution. 2 Under protection, the reaction solution was heated to reflux for 12 hours, and the reflux reaction temperature was 139°C. The reaction precipitate was centrif...
Embodiment 2
[0026]Dissolve 8g of glucose in 40mL of ethylene glycol and 2 Under protection, keep warm at 130°C for 5 hours, so that the color of the ethylene glycol solution changes from colorless to light yellow, which indicates that ethylene glycol glucoside surfactants are formed in the ethylene glycol solution, and finally a light yellow solution A is obtained. 15.7 mg of graphene oxide was ultrasonically dispersed in solution A to obtain solution A containing graphene oxide. Take 0.06mol lithium hydroxide (LiOH·H 2 O) Dissolve in 30mL deionized water, mix it with solution A and stir evenly to obtain solution B. Take 0.02mol manganese chloride (MnCl 2 ) and 0.02mol phosphoric acid (H 3 PO 4 ) was dissolved in 30mL deionized water to obtain solution C, and solution C was added to solution B to form a reaction solution. 2 Under protection, the reaction solution was heated to reflux for 24 hours, and the reflux reaction temperature was 130°C. The reaction precipitate was centrifuge...
Embodiment 3
[0028] Dissolve 0.7g of glucose in 70mL of ethylene glycol and 2 Under protection, keep warm at 150°C for 1 hour, so that the color of the ethylene glycol solution changes from colorless to light yellow, which indicates that ethylene glycol glucoside surfactants are generated in the ethylene glycol solution, and a light yellow solution A is finally obtained. 31.4 mg of graphene oxide was ultrasonically dispersed in solution A to obtain solution A containing graphene oxide. Take 0.03mol lithium hydroxide (LiOH·H 2 O) Dissolve in 10mL deionized water, mix it with solution A and stir evenly to obtain solution B. Get 0.01mol manganese nitrate (Mn(NO 3 ) 2 ) and 0.01mol phosphoric acid (H 3 PO 4 ) was dissolved in 10mL deionized water to obtain solution C, and solution C was added to solution B to form a reaction solution. 2 Under protection, the reaction solution was heated to reflux for 6 hours, and the reflux reaction temperature was 150°C. The reaction precipitate was ce...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com