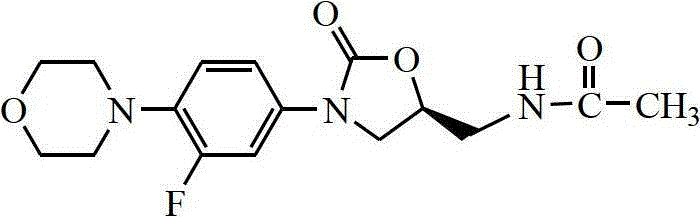
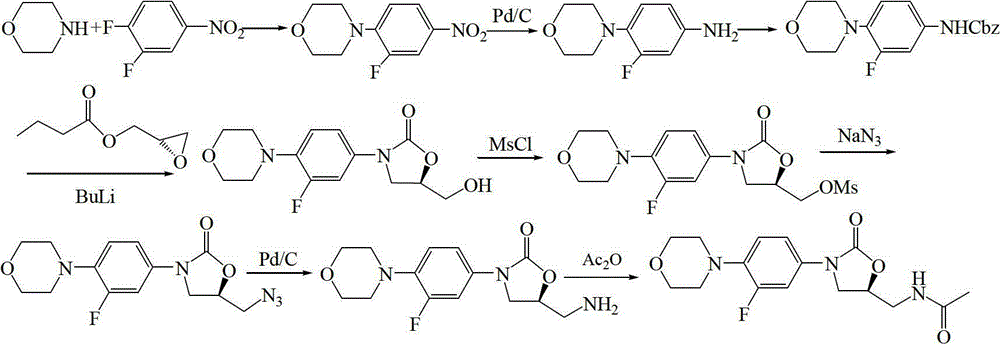
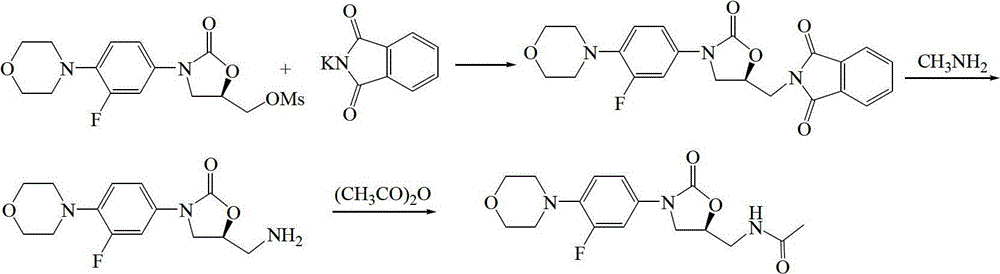
A kind of preparation method of linezolid
A technology of linezolid and triethylamine, which is applied in the field of preparation of linezolid, can solve the problems of low total yield, high cost, difficulty in industrial production of linezolid, etc., achieve mild and easy-to-control reaction conditions, simplify operation, avoid Effect of column separation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 13
[0054] The preparation of embodiment 13-fluoro-4-morpholine nitrobenzene
[0055] In a 3L three-necked flask, install a mechanical stirrer and a thermometer respectively, add 150g (1.72mol) of morpholine, 175g (1.73mol) of triethylamine and 800mL of ethyl acetate, stir well at room temperature, and slowly add 3,4-difluoro 250g (1.57mol) of nitrobenzene was added dropwise within 40min, and stirred at room temperature for 24h, a large amount of yellow solid was formed. TLC showed that the reaction was complete. The reaction mixture was poured into 2.8L of ethyl acetate, fully stirred to dissolve, the solution was washed with water and saturated brine respectively, separated, dried, filtered, and the filtrate was concentrated and crystallized to obtain 351.3 g of a golden yellow solid. The yield is 99.0%.
Embodiment 23
[0056] The preparation of embodiment 23-fluoro-4-morpholine aniline
[0057] In a 3L three-necked flask, add a mechanical stirrer and a thermometer respectively, add 100g (0.44mol) of 3-fluoro-4-morpholine nitrobenzene, 1L of acetone, 3.0g of 10%Pd / C and 137.5g (2.18mol) of ammonium formate ), fully stirred, heated up to 50°C, stirred and reacted for 8 hours, TLC showed that the reaction had been completed, then added 500 mL of acetone, stirred evenly, aged for 2 hours, filtered, and the filtrate was concentrated to dryness to obtain 96 g of off-white solid, the product was not purified. used directly in the next reaction.
Embodiment 3
[0058] The preparation of embodiment 3N-benzyloxycarbonyl-3-fluoro-4-morpholinylaniline
[0059] In a 3L three-necked flask, add 96g of unpurified 3-fluoro-4-morpholine aniline, 1.8L of acetone, 800mL of water and 73.9g (0.88mol) of sodium bicarbonate, stir well, and cool down to 0~5°C in an ice-water bath , and then slowly dropwise added 90.0 g (0.53 mol) of benzyl chloroformate, after the dropwise addition was completed, it was slowly raised to room temperature, and the reaction was stirred overnight. The reaction mixture was poured into ice water to precipitate a solid, which was left to stand for 2 h, filtered, washed with water and ethanol, and dried to obtain 132 g of an off-white solid with a yield of 91.0%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com