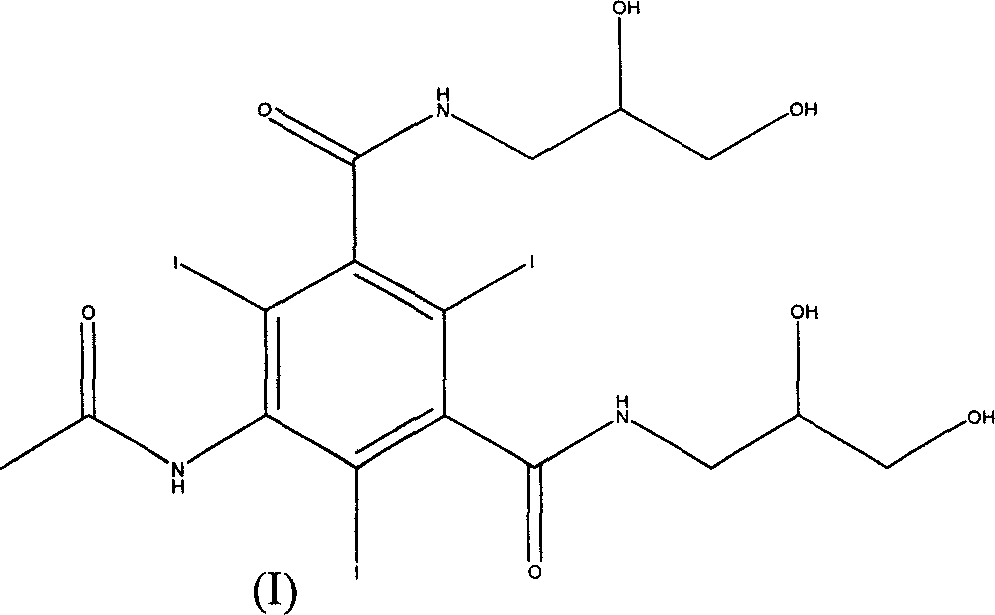
Process for preparing 5-acetamino-N,N'-bis (2,3-dihydroxypropyl)-2,4,6-tri-iodo isophthalamide
A technology of triiodoisophthalamide and acetamido, which is applied in the chemical field, can solve the problems of heavy workload of intermediate separation, refining and baking, easy side reactions between intermediates and products, and impact on product quality, so as to avoid side effects. Responsive, easy to recycle, can be applied mechanically, and the effect of improving product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
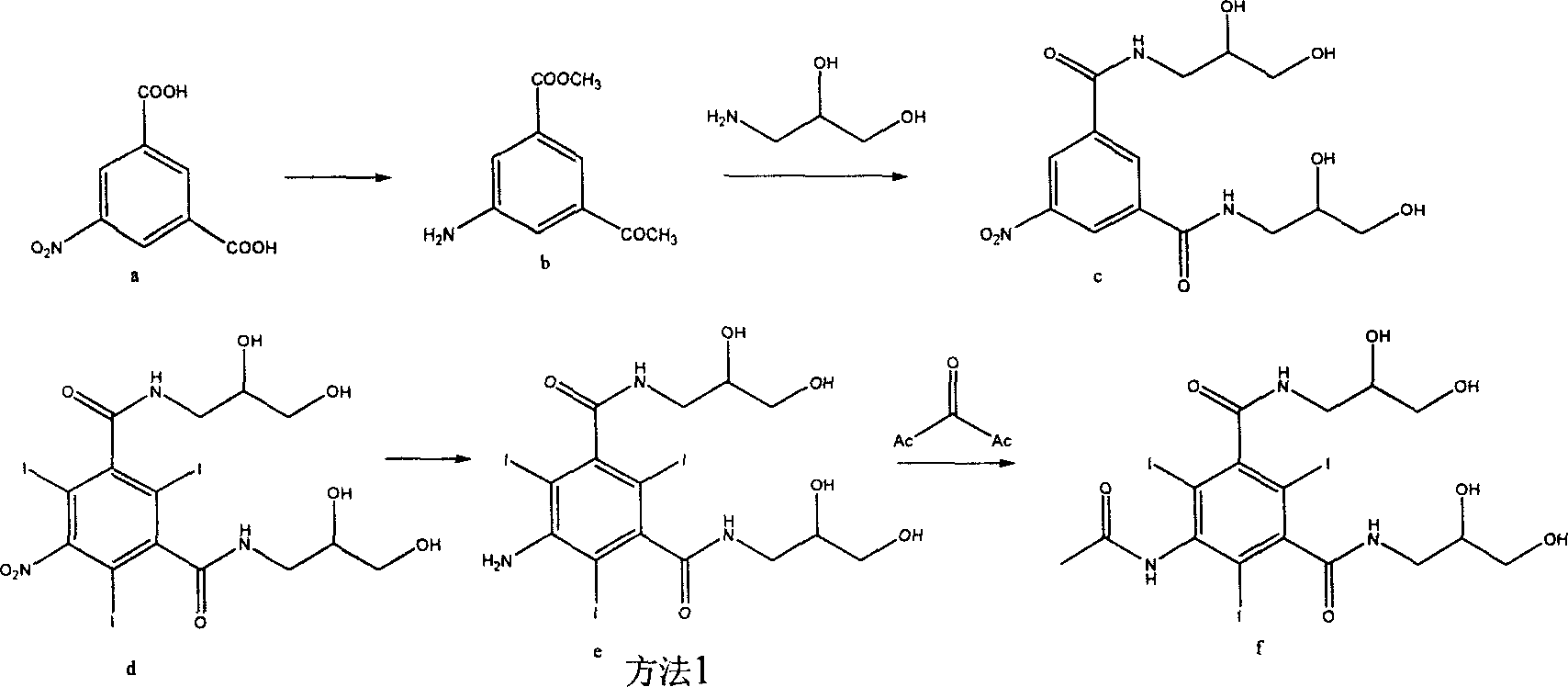
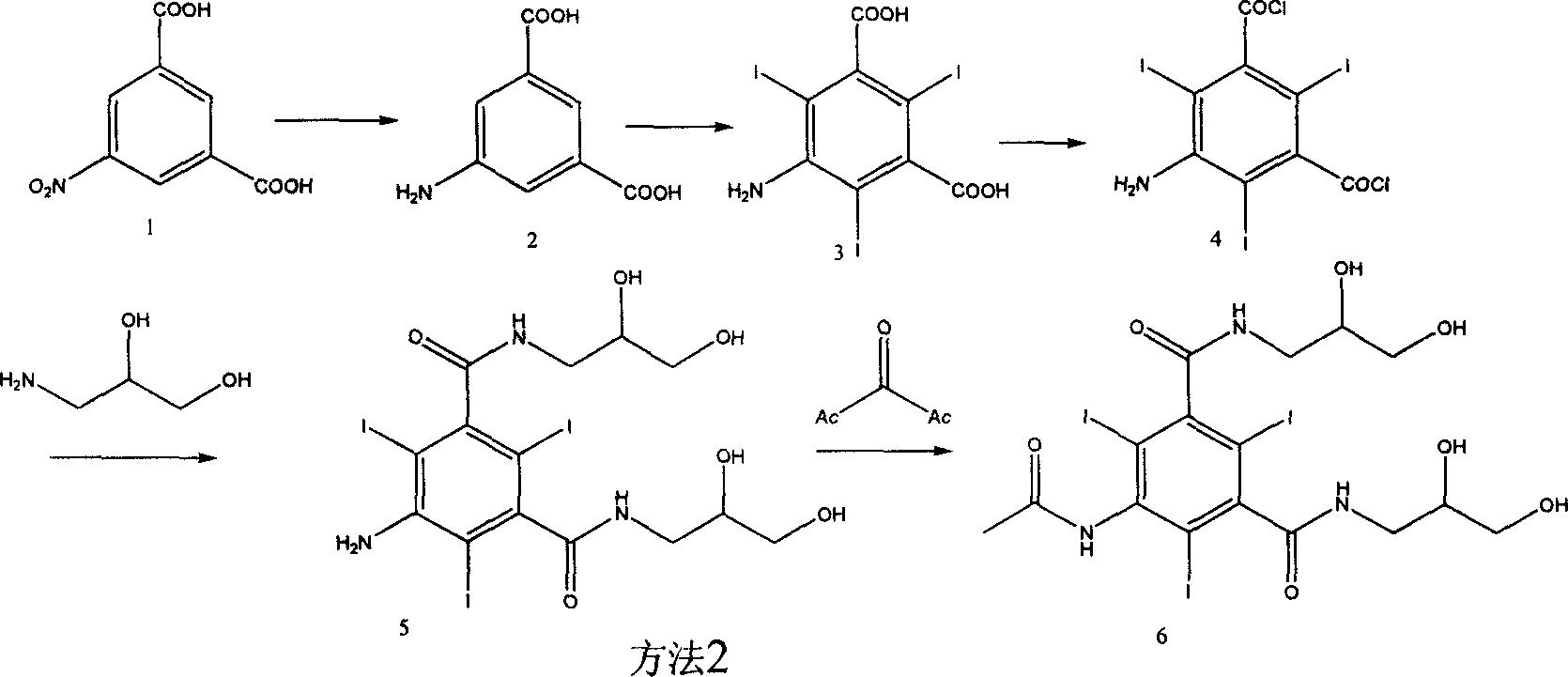
Method used
Image
Examples
Embodiment 1
[0029] The preparation method of embodiment 1,5-acetylamino-N, N'-bis(2,3-dihydroxypropyl)-2,4,6-triiodoisophthalamide, with 5-nitroisophthalic acid As the main starting material, proceed as follows:
[0030] 1) Reduction: Add 120g of reduced iron powder, 38g of sodium chloride and 900ml of deionized water into a 1000mL three-necked bottle. Heat to 90°C and slowly add 42ml of concentrated hydrochloric acid, and heat to reflux for 30 minutes. Stop heating, slowly add 90g of 5-nitroisophthalic acid, heat and reflux for 6 hours after the addition, after the reaction is completed, cool to 65-70°C, adjust the pH value to 10-11 with 35% aqueous sodium hydroxide solution, and Filter iron sludge. The filtrate was adjusted to pH 4-4.5 with concentrated hydrochloric acid, and solids were precipitated, cooled to 5°C, filtered, and dried to obtain 75 g of compound (2), with a molar yield of 97.13%, HPLC: 98%.
[0031] 2), iodination: add 78.4g of compound (2) and 1600ml of deionized wa...
Embodiment 2
[0035] The preparation method of embodiment 2, 5-acetylamino-N, N'-bis(2,3-dihydroxypropyl)-2,4,6-triiodoisophthalamide differs from Example 1 in that , In step 3), 250ml of dioxane was used to replace 250mL of tetrahydrofuran to obtain 84.2g of product with a yield of 55.95%, HPLC: 97.8%.
Embodiment 3
[0036] Embodiment 3, the preparation method of 5-acetylamino-N, N'-bis(2,3-dihydroxypropyl)-2,4,6-triiodoisophthalamide: the difference from Example 1 is , In step 3), 300ml of cyclohexane was used to replace 250mL of tetrahydrofuran to obtain 76g of product with a yield of 50.51%, HPLC: 96.7%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com