Ruthenium complex and application thereof
A ruthenium complex and reaction technology, applied in the field of ruthenium complexes, can solve the problems of large toxic and side effects, low selectivity, etc., and achieve the effects of high selectivity, good anticancer activity, and low normal cytotoxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
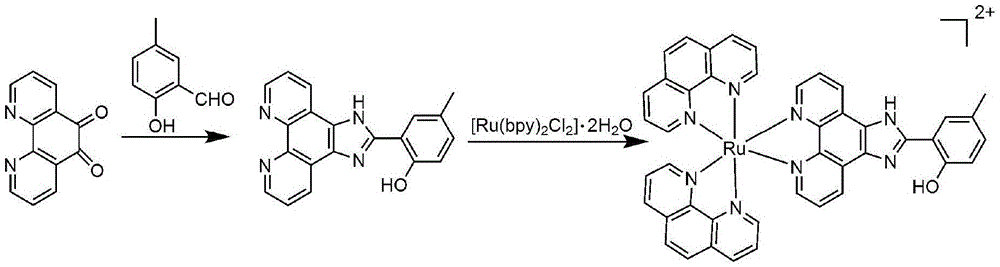
[0037] Embodiment 1 mononuclear ruthenium complex [Ru(phen) 2 ( HIPMP )] (ClO 4 ) 2 Synthesis
[0038] (1) Dissolve 1,10-phenanthroline-5,6-dione, ammonium acetate and 5-methyl salicylaldehyde in an appropriate amount of glacial acetic acid at a ratio of 1:20:1, inert The mixture was mixed and heated to reflux for 4 hours under gas protection. After the mixture was cooled to room temperature, water was added, neutralized with 25% ammonia water, filtered with suction, and the yellow precipitate was washed with water and diethyl ether to obtain the corresponding crude product. The crude product was dissolved with a small amount of ethanol, packed into a column with silica gel (60-100 mesh), and ethanol was used as eluent, and the yellow fraction was collected and rotary evaporated to obtain a yellow powder 2-(1 H- imidazo-[4,5-f][1,10]phenanthrolin-2-yl)-4-methylphenol (the intermediate HIPMP ). Yield: 83%. Anal. Calcd for C 20 h 14 N 4 O:C,73.61;H,4.32;...
Embodiment 2
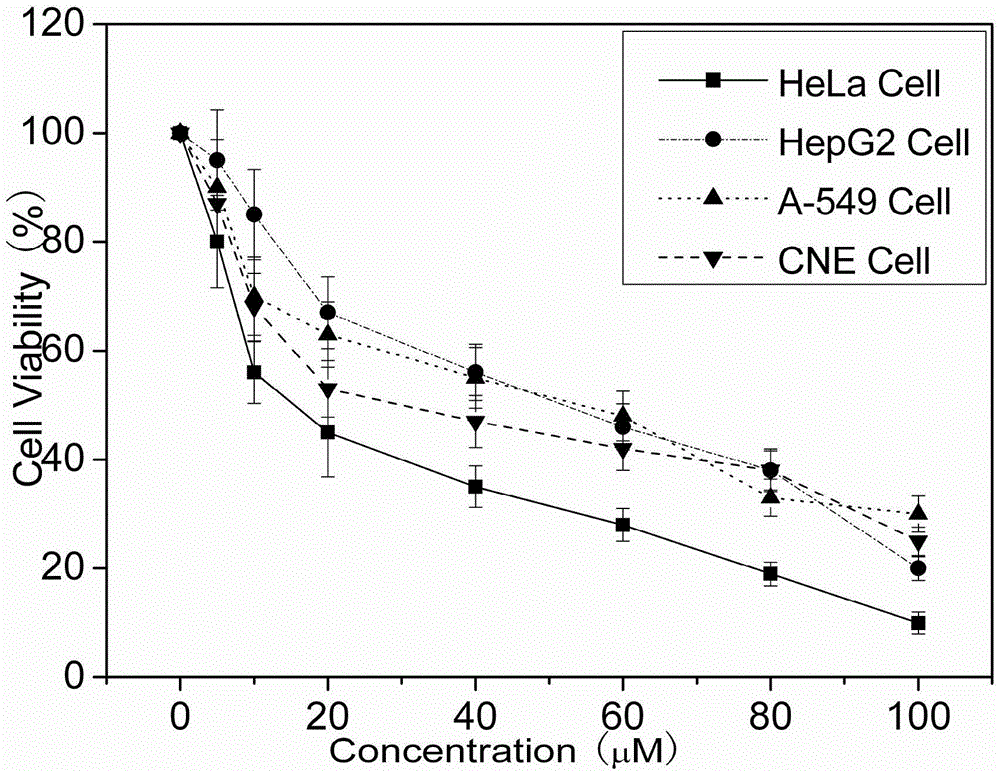
[0041] Example 2 Inhibitory effect of mononuclear ruthenium (II) complexes on proliferation of tumor cells HeLa, A549, HepG2 and CNE-1
[0042] Cytotoxicity test: MTT method was used to study the in vitro toxicity test of the complex. First, place the experimental cells at 37°C, 5.0% CO 2 Grow to the logarithmic phase in the incubator, digest the cells with 0.25% trypsin, and adjust the concentration of the cell suspension so that the cell density is about 1×10 4 Cells / mL, 100mL per well was inoculated in a 96-well plate, and the cell density was about 3-5×10 3 pc / well, placed at 37℃, 5%CO 2 cultured in an incubator for 24 h. Change the medium, add drugs with different concentration gradients, make 3 parallel samples for each concentration, set up blank zero-adjustment group (medium, MTT, DMSO), blank group (medium, cells, drug dissolution medium with the same concentration, MTT, DMSO), positive control group (medium, cells, different concentrations of cisplatin, MTT, DM...
Embodiment 3
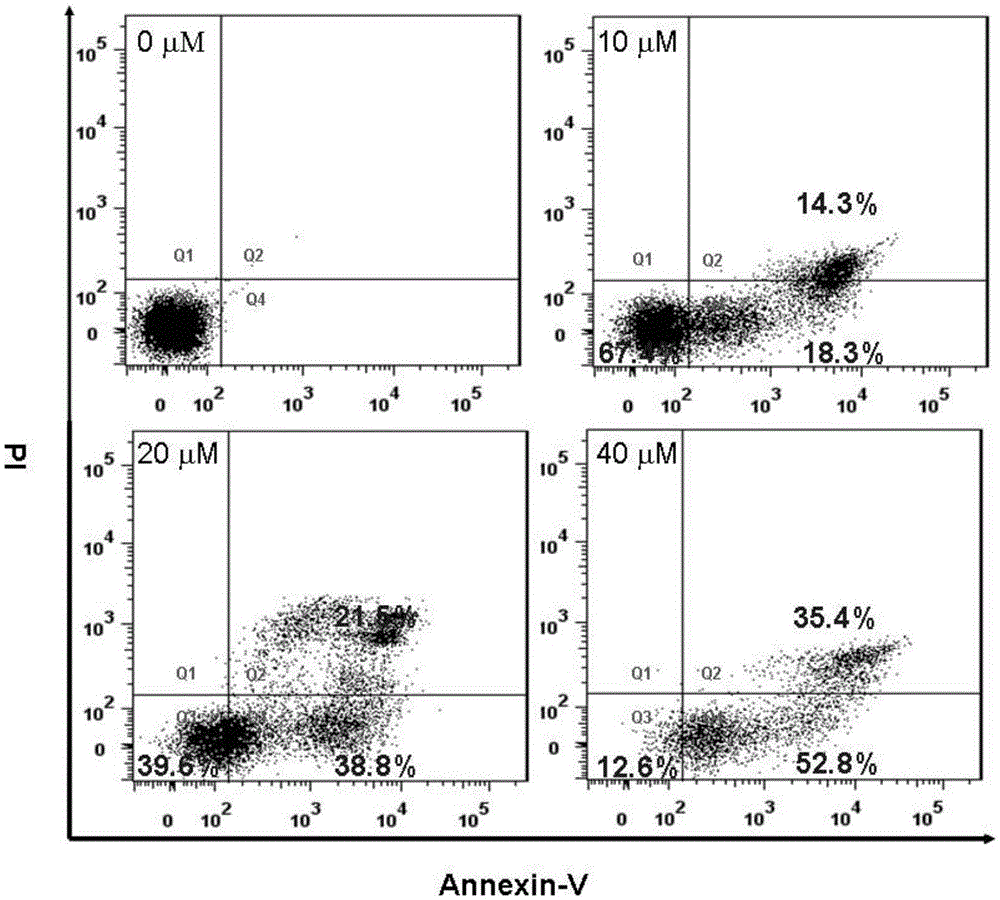
[0047] Example 3 Mononuclear ruthenium (II) complex using AnnexinV and PI double staining method to study HeLa cell apoptosis experiment
[0048] Alexafluor ? 488annexinV / PI double staining method is a semi-quantitative cell apoptosis analysis method detected by flow cytometry (Vermes, C.Haanen, H.Steffens-Nakken and C.Reutellingsperger, J.Immunol.Methods, 1995,184,39 -51.), the specific experimental steps are as follows: collect logarithmic phase cells, adjust the concentration of cell suspension, 1 × 10 per well in a 6-well plate 5 cells. Placed at 37°C, 5% CO 2 Cultivate for about 24 hours in an incubator. Change the medium, add 1mL of different concentrations (10, 20, 40μM) of drugs, three parallel wells for each concentration, and set a blank control well with the same concentration of drug dissolution medium at the same time, and continue to incubate for 24h. Carefully suck off the supernatant, collect the cells by digesting with 0.25% trypsin, and adjust the conce...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap