Method for Continuously Preparing Pyranotetrahydroindolizine Using Microflow Field Reaction
A microfluidic field and reaction technology, applied in organic chemistry and other directions, can solve the problems of difficult control of heat release, potential safety hazards, and high operational requirements, so as to improve controllability and safety, reduce by-product formation, and improve reaction Yield effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
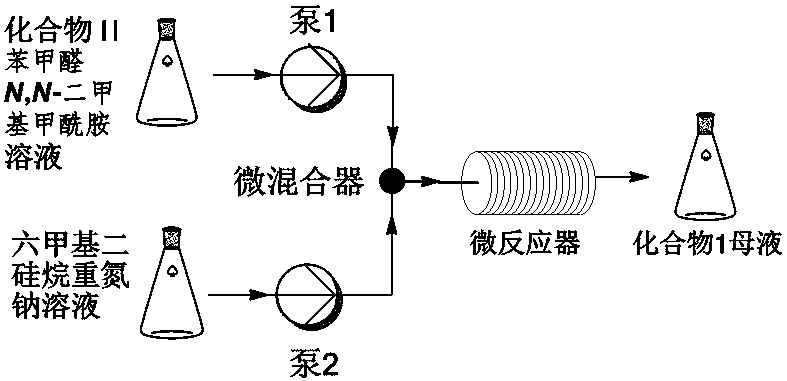
[0024] according to figure 1 The flow chart of, will concentration be the compound II of 6wt% and the benzaldehyde of 2.8wt% N,N -Dimethylformamide solution and the tetrahydrofuran solution of bis(trimethylsilyl) sodium amide with a concentration of 0.6 mol / L are respectively pumped into the mixer as mobile phase and mixed quickly, and the flow rate is controlled so that compound II, benzaldehyde and bis( The molar ratio of sodium trimethylsilyl)amide is 1:1.3:1.4. The material passes through the -40°C reactor with a residence time of 10 minutes. The reaction solution was quenched by flowing into water. After the completion, the pH was adjusted to 1~2 with 5M hydrochloric acid solution, and crystallized at 0°C to obtain the product pyranoindolizine (I) with a yield of 89%.
Embodiment 2
[0026] according to figure 1 The flowchart of, the concentration is the compound II of 4wt% and the benzaldehyde of 1.7wt% N,N -Dimethylformamide solution and the tetrahydrofuran solution of bis(trimethylsilyl) sodium amide with a concentration of 0.5 mol / L are respectively pumped into the mixer as mobile phase and mixed quickly, and the flow rate is controlled so that compound II, benzaldehyde and bis( The molar ratio of sodium trimethylsilyl)amide is 1:1.2:1.4. The material passes through the -45°C reactor with a residence time of 12 minutes. The reaction solution was quenched by flowing into water. After the completion, the pH was adjusted to 1~2 with 5M hydrochloric acid solution, and crystallized at 0°C to obtain the product pyranoindolizine (I) with a yield of 87%.
Embodiment 3
[0028] according to figure 1 The flow chart of, will concentration be the compound II of 7wt% and the benzaldehyde of 3.3wt% N,N -Dimethylformamide solution and the tetrahydrofuran solution of bis(trimethylsilyl) sodium amide with a concentration of 0.4 mol / L are respectively pumped into the mixer as mobile phase and mixed quickly, and the flow rate is controlled so that compound II, benzaldehyde and bis( The molar ratio of sodium trimethylsilyl)amide is 1:1.3:1.4. The material passes through the -35°C reactor with a residence time of 7 minutes. The reaction solution was quenched by flowing into water, and then adjusted to pH 1-2 with 5M hydrochloric acid solution, and crystallized at 0°C to obtain the product compound (I) with a yield of 90%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com