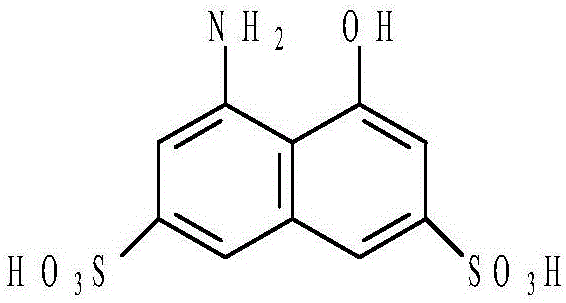
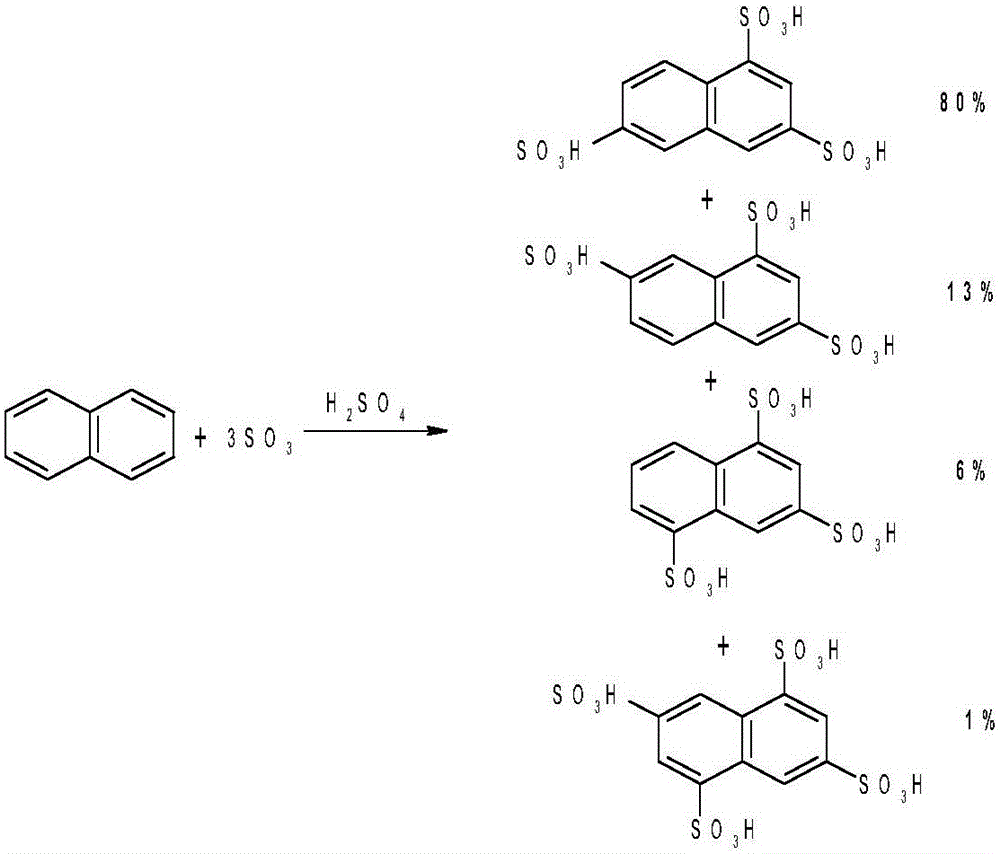
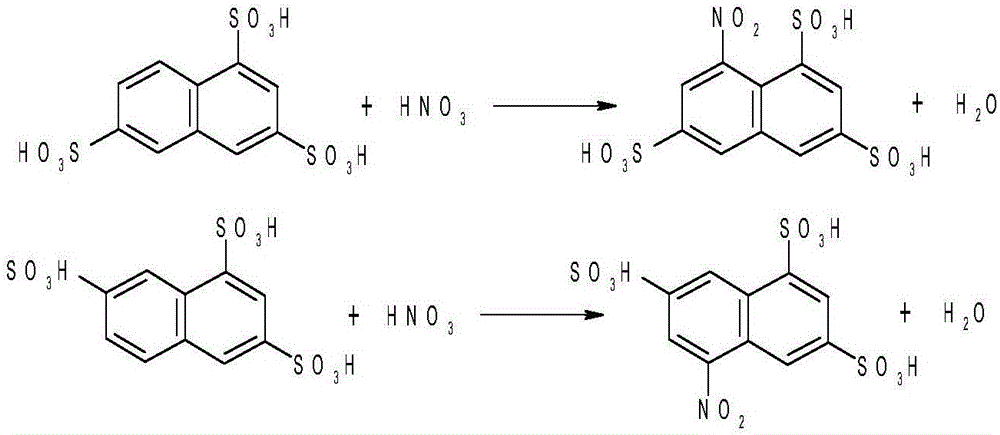
Method for preparing energy-efficient H-acid
A high-efficiency, energy-saving, and effective technology, which is applied in the preparation of sulfonic acid, sulfonate, organic chemistry, etc., can solve the problems of large discharge of three wastes, low production capacity, and long process routes, so as to reduce economic costs and improve production efficiency Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0028] The technical solution of the present invention will be described in detail below in conjunction with the embodiments.
[0029] A kind of preparation method of the H-acid with high efficiency and energy-saving effect of the embodiment of the present invention comprises the following process:
[0030] Step 10) performing sulfonation treatment to obtain sulfonated compounds;
[0031] Step 20) Nitrate the sulfonated compound obtained in step 10) to obtain the nitrated compound;
[0032] Step 30) performing denitrification treatment on the nitrated compound obtained in step 20) to obtain the denitrified compound;
[0033] Step 40) extracting the denitrated substance obtained in step 30) to obtain dilute sulfuric acid and an extraction mixture, and performing back extraction on the extraction mixture to obtain a nitro-T acid solution;
[0034] Step 50) performing reduction treatment on the nitro-T acid solution obtained in step 40) to obtain an amino-T acid solution;
[0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com