A method for deep removal of cobalt from zinc sulfate leaching solution
A zinc sulfate solution and leaching solution technology, applied in the field of cobalt removal, can solve the problems of low purification depth of antimony salt and copper salt, unsatisfactory cobalt removal effect, highly toxic gas of arsenic hydrogen, etc. High efficiency and low cost effect of cobalt
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
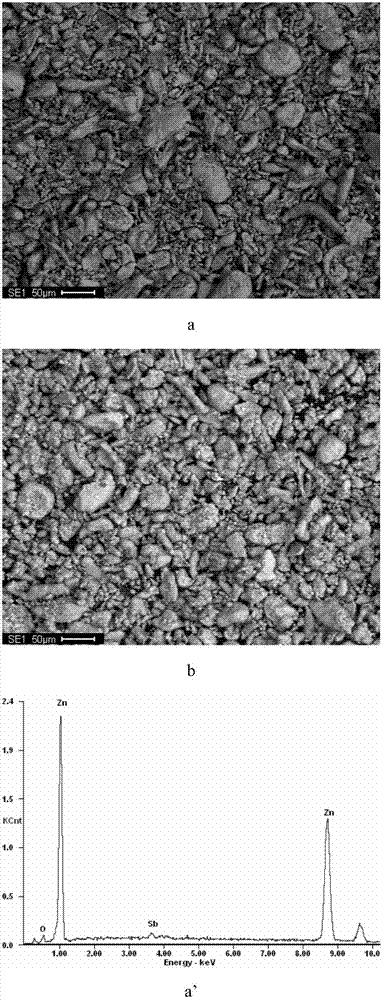
[0039] First, weigh 0.2155g Sb 2 o 3 And disperse in water, prepare 100mL activation solution containing 1.8g / L antimony, adjust the pH of the solution to 3.2 with dilute sulfuric acid, then add 12.5g zinc powder (-200 mesh) according to the liquid-solid ratio of 8, and stir at room temperature The reaction was terminated after activation for 55 min, and the filtrate and filter residue were collected by filtration. The filter residue is used as a cobalt removal agent, and the filtrate is returned for use.
[0040] Next, measure 5L of zinc sulfate leaching solution containing 27.6mg / L of cobalt, 134g / L of zinc, and pH of 4.4 in the reactor, start stirring and heat up to 85°C, add the above-mentioned activated zinc powder, and keep warm for reaction After 60 minutes, after filtering, a zinc sulfate solution containing 0.68 mg / L of cobalt can be obtained, and the cobalt removal rate is 97.38%.
[0041] The microscopic morphology and EDS analysis results of antimony activated z...
Embodiment 2
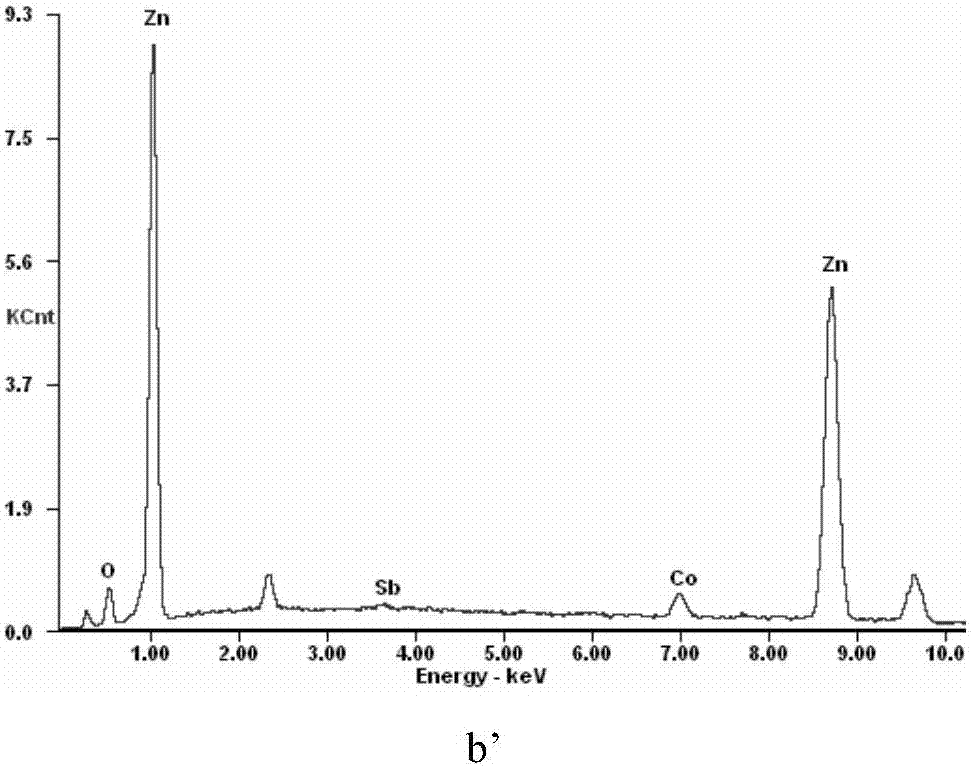
[0047] First, weigh 0.2155g CuSO 4 ·5H 2 O and dissolved in water, prepared into 150mL activation solution containing 3.6g / L copper, adjusted the pH of the solution to 2.7 with dilute sulfuric acid, then added 10g of zinc powder (-200 mesh) according to the liquid-solid ratio of 15, and stirred at room temperature The reaction was terminated after activation for 50 min, and the filtrate and filter residue were collected by filtration. The filter residue is used as a cobalt removal agent, and the filtrate is returned for use.
[0048] Next, measure 6.4L of zinc sulfate leaching solution containing 27.6mg / L of cobalt, 134g / L of zinc, and pH of 4.4 in the reactor, start stirring and heat up to 80°C, add the above-mentioned activated zinc powder, and keep warm After reacting for 75 minutes, a zinc sulfate solution containing 0.76 mg / L of cobalt can be obtained after filtering, and the cobalt removal rate is 97.62%.
[0049] The microscopic morphology and EDS analysis results of...
Embodiment 3
[0051] First, successively weigh 0.1437g Sb 2 o 3 and 0.9375g CuSO 4 ·5H 2 O and dispersed in water, prepared into 120mL activation solution containing 1g / L antimony and 2g / L copper, adjusted the pH of the solution to 2.4 with dilute sodium hydroxide, then added 12g zinc powder (-200 order), the reaction was terminated after stirring and activating at room temperature for 35 minutes, and the filtrate and filter residue were collected by filtration. The filter residue is used as a cobalt removal agent, and the filtrate is returned for use.
[0052] Next, measure 7.2L of zinc sulfate leaching solution containing 27.6mg / L of cobalt, 134g / L of zinc, and pH of 4.4 in the reactor, start stirring and heat up to 75°C, add the above-mentioned activated zinc powder, and keep warm After reacting for 45 minutes, a zinc sulfate solution containing 0.55 mg / L of cobalt can be obtained after filtering, and the cobalt removal rate is 98.44%.
[0053] The microscopic morphology and EDS ana...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com