Isoquinoline-1,3-(2h,4h)-dione substituted with methyl isobutyrate group and its preparation method and use
A 3-co2et, 3-cl technology, applied in organic chemistry, antineoplastic drugs, drug combination, etc., to achieve the effect of high yield, mild reaction system and simple conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
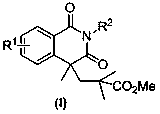
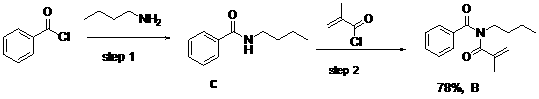
[0039] According to the preparation method in the foregoing examples, the following can also be prepared N -Isopropionyl- N -Alkylbenzamide derivatives (II), the general reaction formula is as follows:
[0040]
[0041] R in the general reaction formula 1 , R 2 Can be as shown in the following groups:
[0042] 1: R 1 = H, R 2 = n -Bu
[0043] 2: R 1 = H, R 2 = i -Pr
[0044] 3: R 1 = 4-CF 3 , R 2 = n -Bu
[0045] 4: R 1 = 4-SMe, R 2 = i -Pr
[0046] 5: R 1 = 4-F, R 2 = n -Bu
[0047] 6: R 1 = 4-Me,R 2 = n -Bu
[0048] 7: R 1 = 4-Me,R 2 = CH 2 Ph
[0049] 8: R 1 = 2-pyridyl, R 2 = n -Bu
[0050] 9: R 1 = 2-Me,R 2 = i -Pr
[0051] 10: R 1 = 4-Br, R 2 = n -Bu
[0052] 11: R 1 = 4-OMe, R 2 = i -Pr
[0053] 12: R 1 = 3-Cl, R 2 = n -Bu
[0054] 13: R 1 = 3-CO 2 Et, R 2 = Me
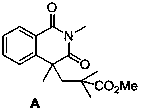
[0055] Preparation of target product A
[0056] The target product provided by the invention contains the isoquinoline-1,3(2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com