Fused 1,4-dihydrodioxin derivatives as inhibitors of heat shock transcription factor 1
A fused, selected technique, for use in conditions or diseases, in the field of preparation of compounds as defined herein, capable of solving problems such as inactivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
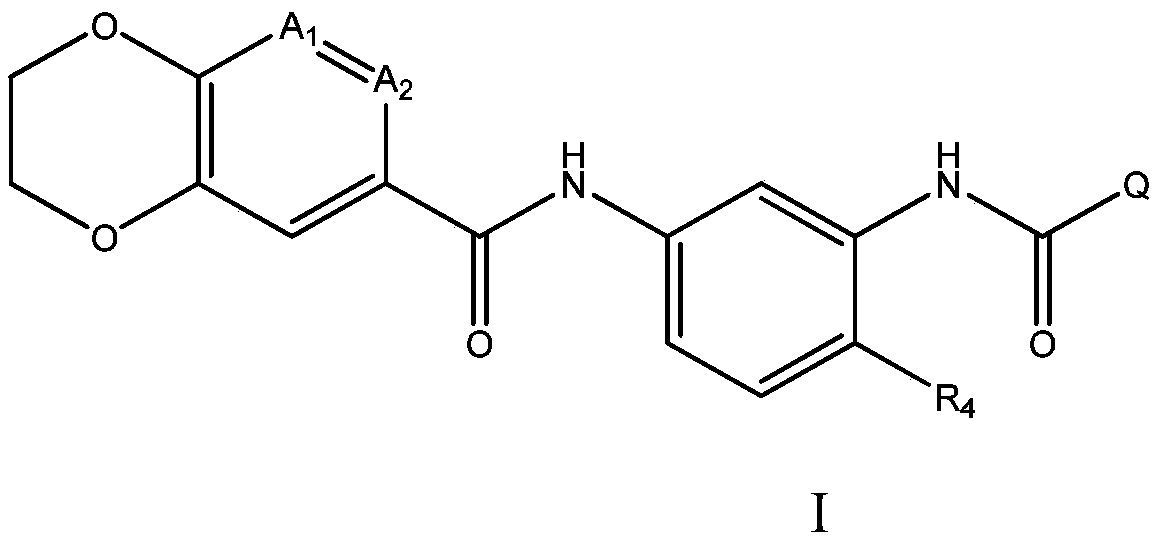
[0786] Example 1, N-(5-(2,3-dihydrobenzo[b][1,4]dioxin-6-amido)-2-methylphenyl)quinoline-6- Formamide
[0787] 2-(7-Aza-1H-benzotriazol-1-yl)-1,1,3,3-tetramethyluronium hexafluorophosphate (HATU, 3.34 g, 8.79 mmol) was added to 6- A solution of quinolincarboxylic acid (1.34 g, 7.74 mmol) and N,N-diisopropylethylamine (DIEA, 2.76 mL, 15.8 mmol) in dry DMF (40 mL). The reaction mixture was stirred for 6 min, after which compound 2 (2.00 g, 7.03 mmol) was added. The reaction mixture was stirred overnight at rt, diluted with water and the resulting precipitate was isolated by filtration, washed with water and dried to afford the title compound (3.09 g, 100%) as an off-white solid. 1 H NMR(500MHz,DMSO)δ10.18(s,1H),10.08(s,1H),9.02(dd,J=4.2,1.7Hz,1H),8.67(d,J=2.0Hz,1H),8.54 (dd, J=8.4,1.9Hz,1H),8.29(dd,J=8.8,2.1Hz,1H),8.15(d,J=8.8Hz,1H),7.88(d,J=2.2Hz,1H) ,7.65(dd,J=8.3,4.2Hz,1H),7.59(dd,J=8.2,2.2Hz,1H),7.54(d,J=2.2Hz,1H),7.51(dd,J=8.4,2.2 Hz, 1H), 7.25 (d, J = 8.4Hz, 1H), 6...
Embodiment 2 to Embodiment 48
[0789] According to the procedure used in Example 1, by substituting the appropriate carboxylic acid for 6-quinolinecarboxylic acid, the following compounds were synthesized:
Embodiment 2
[0790] Example 2, N-(5-(2,3-dihydrobenzo[b][1,4]dioxin-6-amido)-2-methylphenyl)isoquinoline-7-methyl Amide
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com