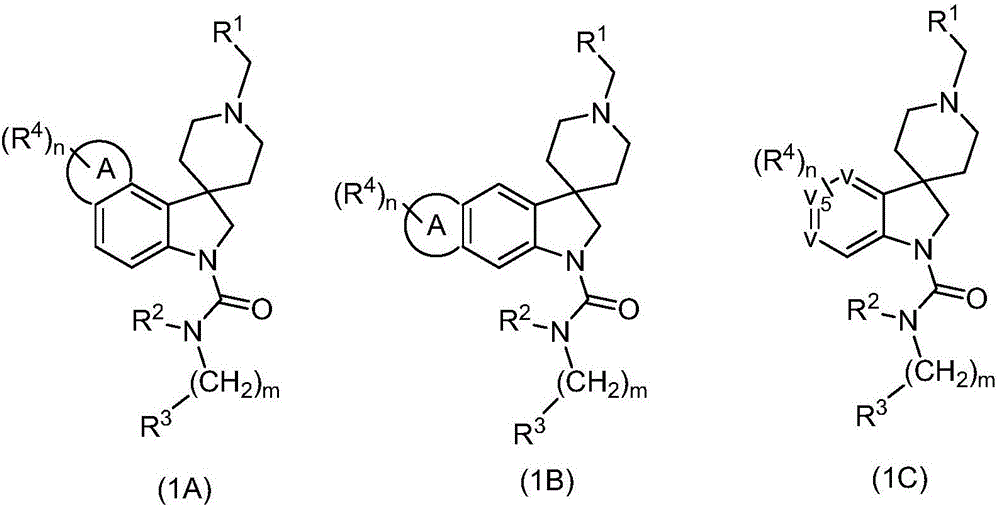
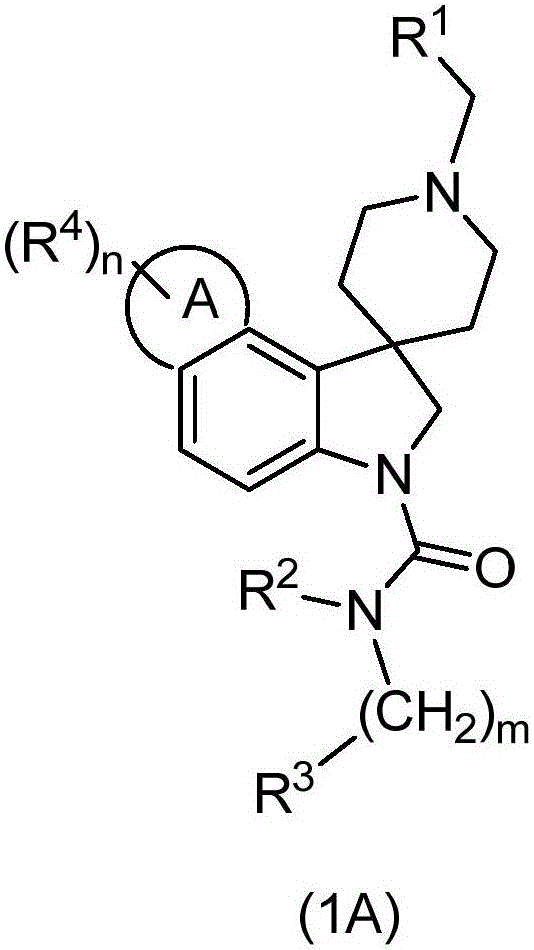
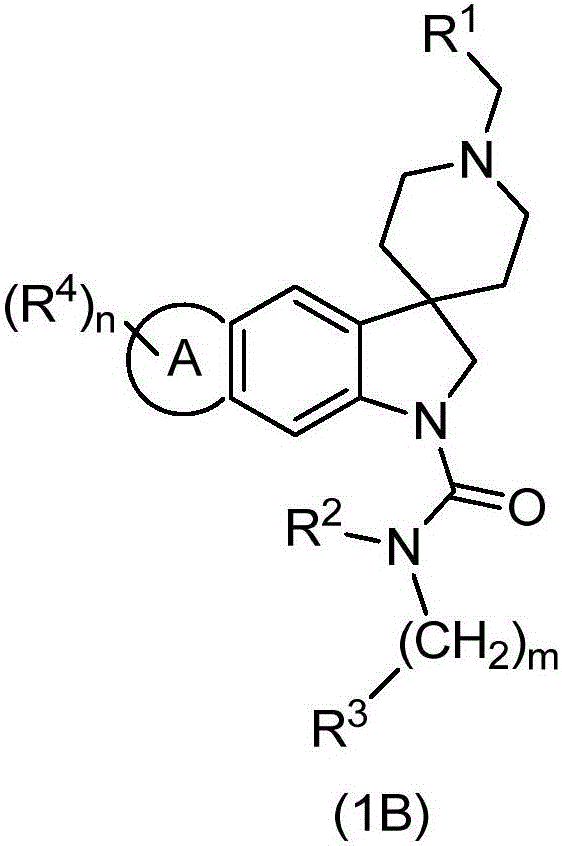
Spiroindoline antiparasitic derivatives
A compound, C1-C6 technology, applied in biocides, drug combinations, anti-infectives, etc., can solve problems such as how to prepare bicyclic compounds that have not been pointed out
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0282] In the preparation of compounds of the present invention, protecting groups may be used to effect protection of remote functional groups of intermediates from undesired reactions. The term "protecting group" or "Pg" refers to a substituent commonly used to block or protect a specific functional group while reacting with other functional groups on the compound. For example, an amine protecting group is a substituent attached to an amine that blocks or protects the amine functionality of a compound or intermediate. Suitable amine protecting groups include: 1-tert-butoxycarbonyl (Boc); acyl groups including: formyl, acetyl, chloroacetyl, trichloro-acetyl, o-nitrophenylacetyl, o-nitrophenyl phenylphenoxyacetyl, trifluoroacetyl, acetoacetyl, 4-chlorobutyryl, isobutyryl, o-nitrocinnamoyl, picolilyl, acyl isothiocyanate, aminocaproyl, benzyl Acyl, etc.; and acyloxy groups, including: methoxycarbonyl, 9-fluorenyl-methoxycarbonyl, 2,2,2-trifluoroethoxycarbonyl, 2-trimethylsilyl...
Embodiment 1
[0414] Example 1. (E)-N-((2-chloropyridin-4-yl)methyl)-1'-(3-(3,4-dichlorophenyl)allyl)-5,7- Dihydrospiro[furo[3,4-f]indole-3,4'-piperidine]-1(2H)-carboxamide
[0415]
[0416] Step 1: 1-((2-Chloropyridin-4-yl)methylcarbamoyl)-1,2,5,7-tetrahydrospiro[furo[3,4-f]indole-3,4 Preparation of tert-butyl '-piperidine]-1'-carboxylate
[0417]
[0418] In a 10 mL vial, to a stirred solution of (2-chloro-pyridin-4-yl)-methylamine hydrochloride (135 mg, 0.758 mmol, 1 equiv) in dichloromethane (6 mL) at 0 °C was added Tris Ethylamine (0.426 mL, 3.034 mmol, 4 eq) followed by 1,1-carbonyldiimidazole (160 mg, 0.986 mmol, 1.3 eq) was added. The reaction was allowed to warm slowly to room temperature and stir for 4 hours. After the starting material was consumed, the reaction was cooled to 0°C and 1,2,5,7-tetrahydrospiro-[furo[3,4-f]indole-3,4'-piperidine]-1'- tert-Butyl formate (Intermediate 1.12b, 201 mg, 0.607 mmol, 0.8 equiv) and stirred at room temperature for 16 hours. After c...
Embodiment 2
[0423] Example 2: (E)-1'-(3-(4-chlorophenyl)allyl)-N-((2-fluoropyridin-4-yl)methyl)-5,7-dihydrospiro [Furo[3,4-f]indole-3,4'-piperidine]-1(2H)-carboxamide
[0424]
[0425] Example 2 was prepared analogously to Example 1 except that (2-fluoro-pyridin-4-yl)-methylamine hydrochloride was used in step 1 instead of (2-chloro-pyridin-4-yl) - methylamine hydrochloride and replace (E)-3-(3,4-dichloro-phenyl)-propenal with (E)-3-(4-chloro-phenyl)-propenal. 1H NMR (400MHz, DMSO) δ: 1.62(d, J=1.216Hz, 2H), 1.82-1.85(m, 2H), 2.08(t, J=11.24Hz, 2H), 2.90(d, J=11.44Hz, 2H), 3.14(d, J=6.4Hz, 2H), 3.89(s, 2H), 4.38(d, J=5.64Hz, 2H), 4.90(d, J=4.4Hz, 4H)6.34-6.41(m ,1H),6.56(d,J=15.88Hz,1H),7.10(d,J=5.64Hz,2H),7.30(d,J=4.76Hz,1H),7.37(d,J=8.46Hz,2H ), 7.45-7.50 (m, 3H), 7.74 (s, 1H), 8.16 (d, J=5.08Hz, 1H). LC-MS (m / z): 532.8 (M+H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com