Medicine for treating or preventing drug-resistant relapse of acute lymphoblastic leukemia and its application
A technology for acute lymphocytes and leukemia, which is applied in the field of drugs for the treatment or prevention of drug-resistant relapse of acute leukemia, and can solve the problems of lack of detection and treatment methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] Detection of PRPS1 gene mutations in samples:
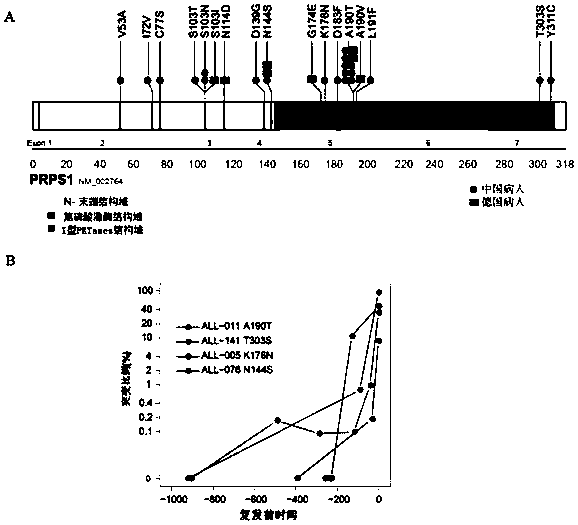
[0071] Using whole-exome sequencing technology, PRPS1 was sequenced on the first-onset, remission and relapse samples of 16 groups of children with ALL and 144 relapse samples.
[0072] Quality control of samples: The quality of samples is directly related to the reliability of sequencing results. The following three methods are used to ensure the quality of samples used for deep sequencing and subsequent verification: ① Leukemic cells in the bone marrow can account for all of the leukemia cells at the onset and recurrence of leukemia. For more than 90% of nucleated cells, the remaining normal granulocytes and nucleated red blood cells can be removed by Ficoll density gradient centrifugation, so that high-purity leukemia cells can be obtained for subsequent DNA extraction and sequencing; ②The combination of multicolor fluorescent antibodies can Distinguish between leukemia cells and normal bone marrow hematopoietic cells. Fo...
Embodiment 2
[0105] Detection of mutations in genes related to purine metabolism pathway in samples
[0106] Purine metabolism-related enzymes HGPRT, IMPDH, NT5C2, PRPS2, ATIC, ADSL, GART, and PFAS were sequenced in 160 children with relapsed ALL samples by conventional next-generation sequencing technology.
[0107] Sample quality control, preparation, amplification of gene exons are the same as in Example 1
[0108] Sequencing analysis: After the PCR is successful, the target band is sequenced, and the target sequence is compared with the corresponding gene sequence in NCBI to analyze the gene mutation. If a genetic mutation is found in a relapse sample, test a primary sample from the same patient to determine if it is a relapse-specific mutation. The experimental results are shown in Table 4.
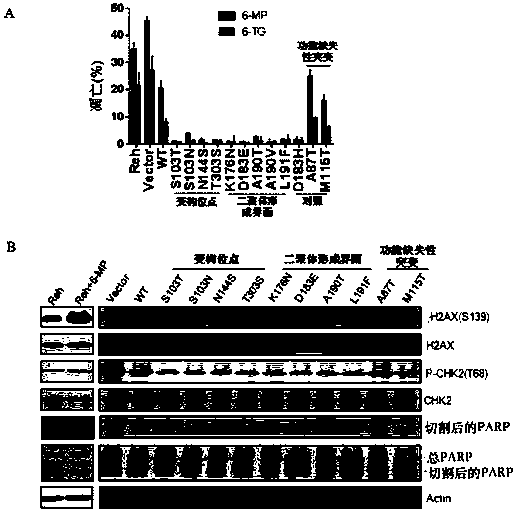
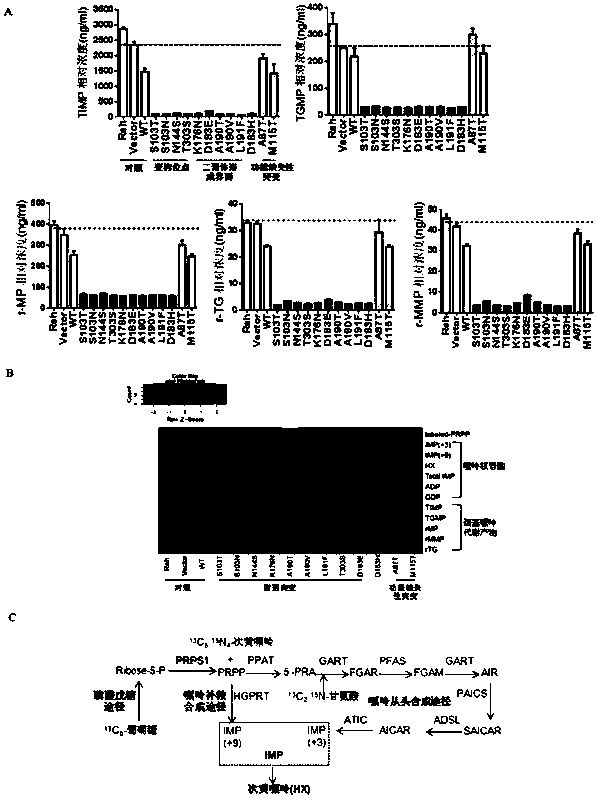
[0109] Through sequencing and sequence comparison, it was found that the purine metabolism-related enzymes PRPS2, ATIC, ADSL, GART, and PFAS all had recurrence-specific mutations.
[0110] Tab...
Embodiment 3
[0114] Construction of PRPS1 prokaryotic expression vector
[0115] 1. Acquisition of the target gene fragment: According to the PRPS1 (gene ID: 5631, NM 002764) sequence provided by NCBI, design the PCR primer sequence of wild-type PRPS1, forward primer: 5'cgcggcagccatATGCCGAATATCAAAATCTTCAG3', reverse primer: 5'gtggtggtgctcgagTTATAAAGGGACATGGCTGAATAGGTA3' (Primer Description: Contains exchange paired bases, restriction sites, and contains the 5' end partial sequence of the target gene for PCR to capture the target gene). Using the extracted cDNA of Reh cells as a template, PRPS1 was captured by PCR, and the size of the PCR product was 957bp, and the product sequence was shown in the sequence table SEQ ID No.1.
[0116] 2. Linearization of the prokaryotic expression vector: treat the pET28a vector with restriction endonuclease NdeI / XhoI, the pET28a vector map is as follows image 3 shown.
[0117] 3. Recombinant plasmid construction: through In-Fusion of clontech company T...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com