Technique of chemical synthesis of producing adenosine
A technology of chemical synthesis and adenosine, which is applied in the field of production of adenosine by chemical synthesis, can solve the problems of reducing production capacity, increasing production costs, difficulties in recycling pyridine and acetic anhydride, etc., and achieves the effect of simplifying the production process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
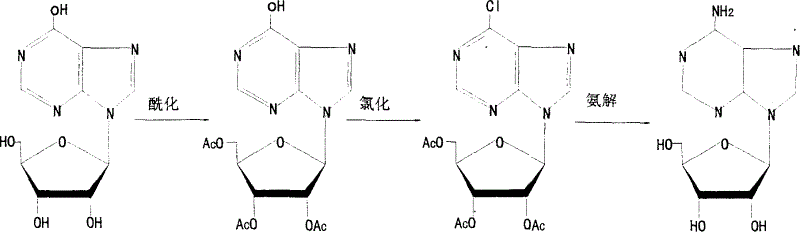
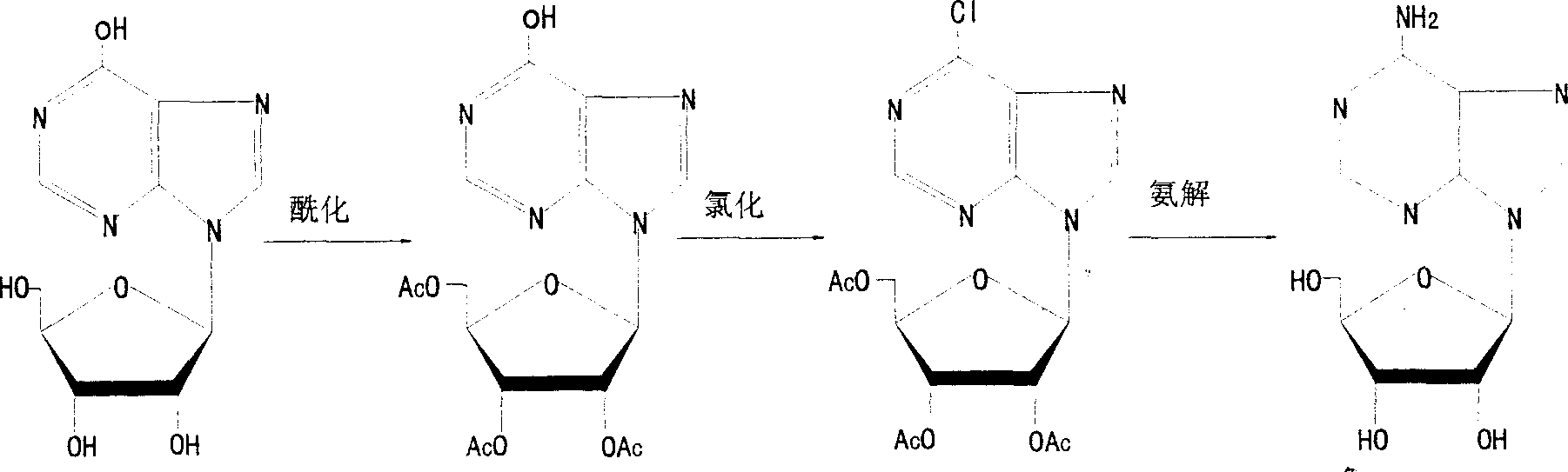
[0039] Synthesis:
[0040] 1. Synthesis of triacetyl inosine
[0041] Add the calculated amount of acetic anhydride and anhydrous sodium acetate into the dry reactor, and when heated to 82°C, add inosine in small amounts and keep the temperature below 120°C. After adding inosine, the reaction was incubated at 120°C for 30 minutes. It was distilled under reduced pressure until no liquid droplet occurred. Cool down to 0°C and centrifuge, wash the crystals with an appropriate amount of cold pure water to pH5. drying. White triacetyl inosine crystals were obtained. The melting point is 242-243°C, the yield is 96%, and the purity is over 99%.
[0042] 2. Halogenation of the 6-hydroxyl group of purine bases
[0043] (1) Add triacetyl inosine and a calculated amount of pyridine into a dry reaction kettle, stir and cool down to below 10°C, then slowly add phosphorus oxychloride dropwise (or flow-in), and heat up to 45-50°C after adding , keep the reaction for 14 hours, and then...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com