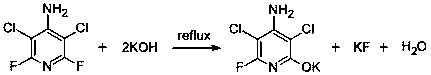A kind of preparation technology of ethylpyridine 4-amino-3,5-dichloro-6-fluoro-2-oxyacetate
A technology of ethyl oxyacetate pyridine and ethyl chloroacetate, which is applied in the field of fine chemicals, can solve the problems of reduced production costs and environmental treatment costs, unfavorable industrial production, and high production costs, so as to reduce environmental treatment costs and improve raw material utilization The effect of low production rate and production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] A preparation process of 4-amino-3,5-dichloro-6-fluoro-2-oxyl ethyl pyridine, characterized in that: comprising the following steps:
[0052] A. Amination
[0053] Add 3,5-dichloro-2,4,6-trifluoropyridine and NMP into the reactor, pass through ammonia gas, after the reaction is complete, filter the reaction solution, and rectify the filtrate to obtain 4-amino-3, 5-dichloro-2,6-difluoropyridine; the waste residue obtained by the filtration is the by-product NH 4 F, the filtrate is a mixture of solvent NMP and 3,5-dichloro-2,4,6-trifluoropyridine. After rectification, NMP and 4-amino-3,5-dichloro-2,6-dichloropyridine are obtained respectively flupyridine;
[0054] B. Hydroxylation
[0055] After adding KOH aqueous solution to the 4-amino-3,5-dichloro-2,6-difluoropyridine obtained in step A, heat to reflux. After the reaction is complete, cool and centrifuge to obtain the mother liquor and solid 4-amino- Potassium 3,5-dichloro-6-fluoropyridin-2-ol;
[0056] C. Mother ...
Embodiment 2
[0062] In this example, on the basis of Example 1, in step A, the amount of ammonia gas introduced is 2.1 times the amount of 3,5-dichloro-2,4,6-trifluoropyridine.
Embodiment 3
[0064] In this example, on the basis of Example 1, in step A, the rectification condition is 80-140°C, and the fraction collected at 122-125°C / 1Kpa is 4-amino-3,5-dichloro-2 ,6-Difluoropyridine.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com