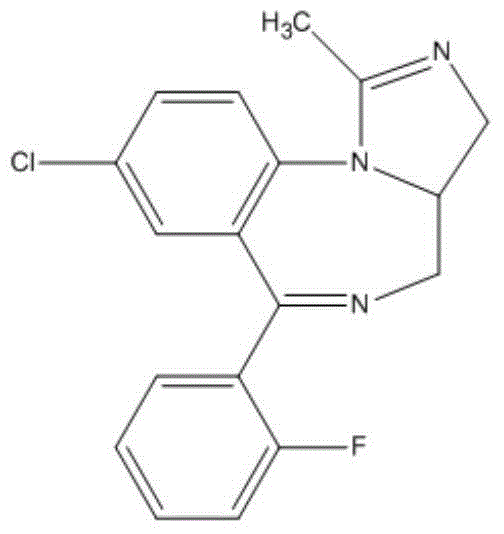
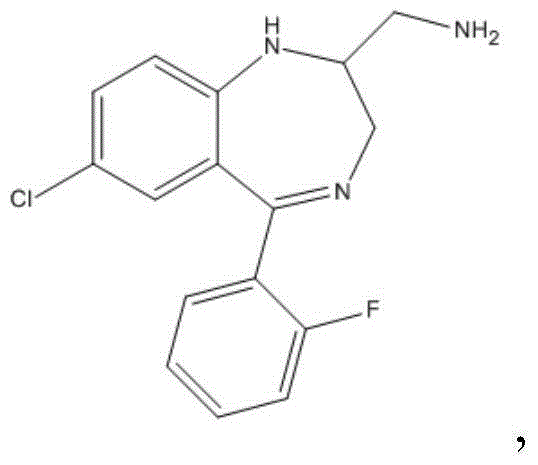
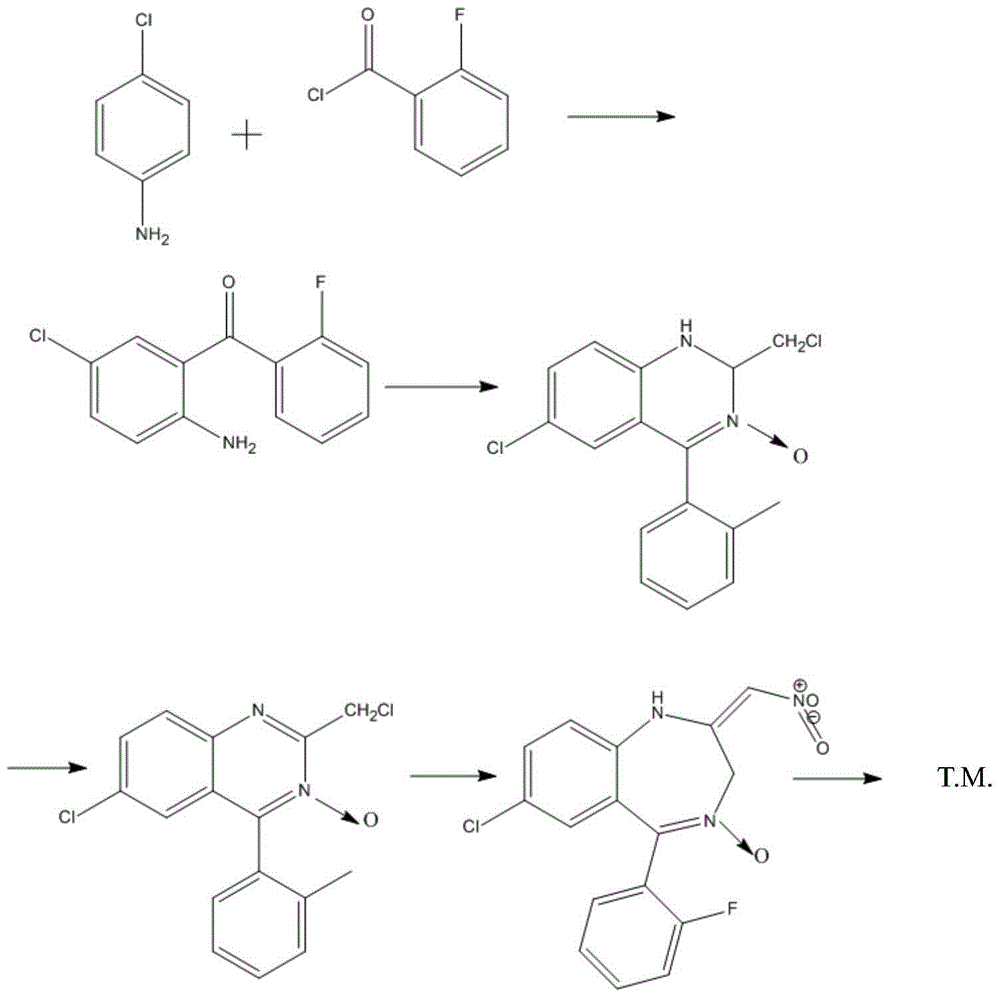
Industrial production method of midazolam derivative
A midazolam, industrial manufacturing technology, applied in the field of industrial manufacturing of midazolam derivatives, can solve the problems of lengthy synthetic routes, lower production efficiency, higher production costs, etc., to increase operational difficulties, increase costs, reduce The effect of three waste discharge
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] The reaction equation is as follows:
[0061]
[0062] Put 500ml of toluene, 250g of A, and 130g of B into the reaction kettle respectively, add 10g of titanium tetrachloride and reflux for 4 hours to complete the reaction. The reaction product is filtered, hydrolyzed, extracted with ether, concentrated and recrystallized with ethyl acetate to obtain 260g of pure product.
[0063] In the above reaction, the molar ratio of compound A to compound B can also be 1:1 or 1:1.2 or 1:1.5 or 1:1.8 or 1:2, etc., and the solvent can also be selected from cyclohexane and other boiling point Solvent in the range of 40-100 ° C, try to use a high molar ratio catalyst in this reaction such as A: catalyst = 1:0.01 or 1:0.1 or 1:0.5 or 1:1.2 or 1:2, the yield is improved To an insignificant extent, excess catalyst can be recycled.
Embodiment 2
[0065]The reaction equation is as follows:
[0066]
[0067] Put 500ml of tetrahydrofuran and 90g of B into the reaction kettle, and slowly add C78.5g dropwise while stirring. After no gas is released, react for 0.5 hours, add A280g, add 10g of titanium tetrachloride and reflux for 2 hours to complete the reaction. Filtration, hydrolysis, extraction with ethyl acetate, concentration and recrystallization with ethyl acetate:ethanol=2:1 gave pure product 280g.
[0068] In the above reaction, the one-pot input method was tried to carry out the reaction, and the yield was slightly lower than the result of the step-by-step one-pot method. The molar ratio of compounds A, B and C can also be 1:1:1 or 1:1.2:1 or 1:1.5:1.1 or 1:1.8:1.5 or 1:2:1.6, and the solvent can also be selected For cyclohexane and other solvents with a boiling point in the range of 40-100°C, try to use a high molar ratio catalyst in this reaction such as A:catalyst=1:0.01 or 1:0.1 or 1:0.5 or 1:1.2 or 1: 2. ...
Embodiment 3
[0070] The reaction equation is as follows:
[0071]
[0072] Put 500ml of tetrahydrofuran and 90g of B into the reaction kettle, add C60g dropwise while stirring, reflux for 2 hours, slowly add A245g in batches, add 20g of titanium tetrachloride and reflux for 8 hours to complete the reaction, and the reaction product is filtered, hydrolyzed, diethyl ether Extraction, concentration and recrystallization from ethanol afforded 277 g of pure product.
[0073] In the above reaction, try to adopt the one-pot input method for the reaction or react the raw materials A and B first and then add the raw material C, and the yield is equivalent to the result of the step-by-step one-pot method. The molar ratio of compounds A, B and C can also be 1:1:1 or 1:1.2:1 or 1:1.5:1.1 or 1:1.8:1.5 or 1:2:1.6 etc. Compound C can also be Selected from such as ethyl acetate, phenyl acetate, benzyl acetate, etc., the solvent can also be selected as cyclohexane and other solvents with a boiling poin...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap