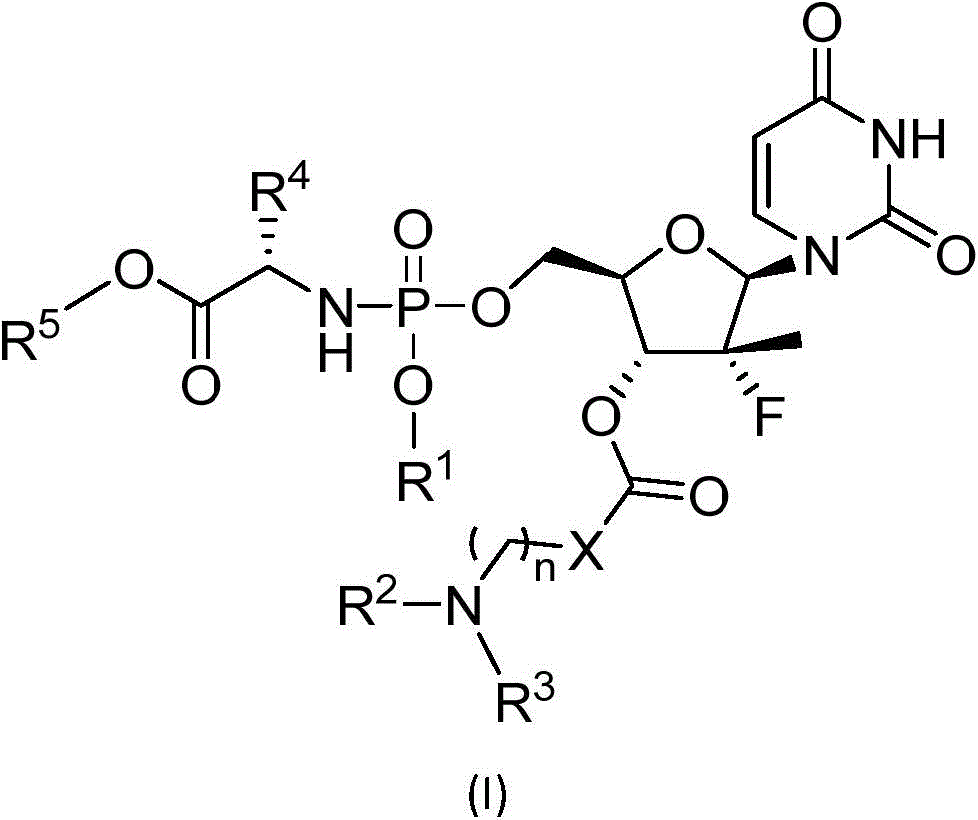
Novel phosphoramidates for treatment of hcv infection
An alkyl and aryl technology, applied in the field of new phosphoramidates for the treatment of HCV infection, can solve the problem of limited curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
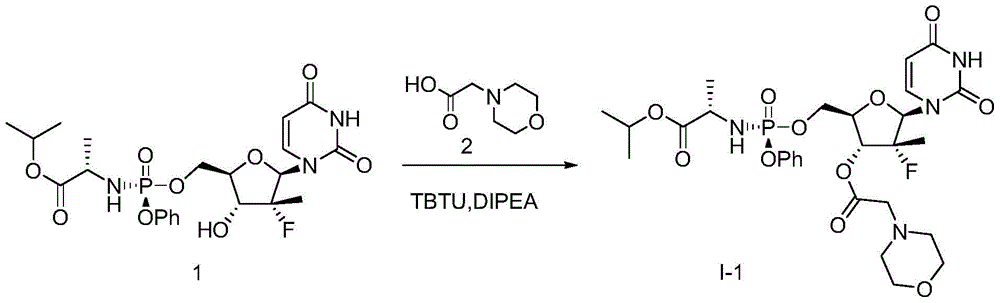
[0091] Example 1. Preparation ((S)-(((2R,3R,4R,5R)-5-(2,4-diketone-3,4-dihydropyrimidine-1(2H)-position)-4- Fluoro‐4‐methyl‐3‐(2‐morpholine acetoxy)tetrahydrofuran‐2‐position)methoxy)(phenoxy)phosphoryl)‐L‐alanine isopropyl ester (I‐1)
[0092]
[0093] Compound 1 (0.30 g, 0.57 mmol), Compound 2 (0.12 g, 0.68 mmol) and DiPEA (0.22 g, 1.70 mmol) were dissolved in DCM (15 mL). After stirring at room temperature for 5 minutes, TBTU (0.22 g, 0.68 mmol) was added and the mixture was stirred for another 20 hrs. The solvent was spin-dried, extracted with EA (100mL), the combined organic layer was washed with water (2x50mL) and saturated brine (50mL) successively, dried over anhydrous sodium sulfate, filtered, concentrated and purified by column chromatography to obtain the target product I‐1 (0.28 g, 76%).
[0094] 1 H‐NMR (400MHz, CDCl 3 )δ: 8.59(s, 1H), 7.50(d, J=8.4Hz, 1H), 7.34(t, J=8.0Hz, 2H), 7.23(d, J=8.4Hz, 2H), 7.18(t, J=7.2Hz, 1H), 6.19(d, J=18.8Hz, 1H), 5.58(d, J=8...
Embodiment 2
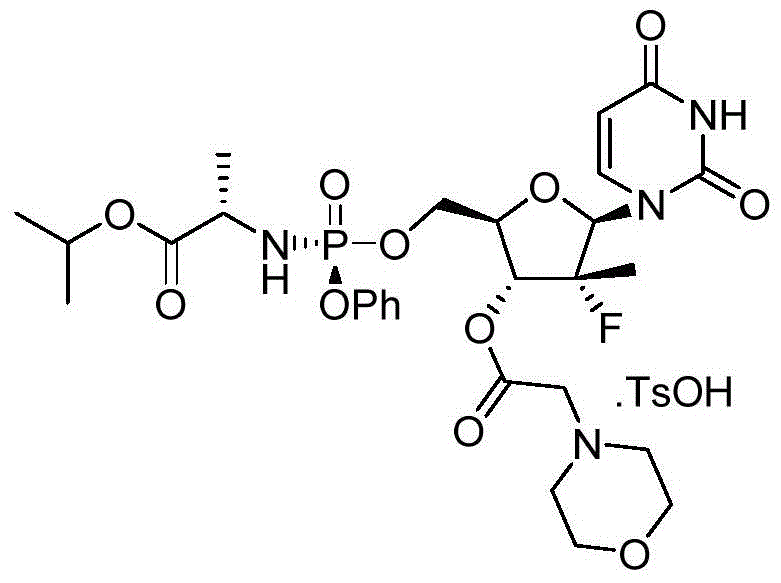
[0095] Example 2. Preparation ((S)-(((2R,3R,4R,5R)-5-(2,4-diketone-3,4-dihydropyrimidine-1(2H)-position)-4- Fluoro‐4‐methyl‐3‐(2‐morpholine acetoxy)tetrahydrofuran‐2‐position)methoxy)(phenoxy)phosphoryl)‐L‐alanine isopropyl ester 4‐methylbenzene Sulfonate (I‐2)
[0096]
[0097] Compound 1-1 (0.500 g, 0.76 mmol) was dissolved in DCM (12 mL), then p-toluenesulfonic acid (0.145 g, 0.76 mmol) was added. The reaction solution was stirred for 0.5 h, then concentrated and recrystallized to obtain the target salt (0.450 g).
[0098] 1 H‐NMR (400MHz, CDCl 3 )δ: 7.71 (d, J = 7.2Hz, 2H), 7.50 (d, J = 7.2Hz, 1H), 7.30‐7.27 (m, 2H), 7.21‐7.14 (m, 6H), 6.05 (brs, 1H ),5.63(d,J=7.8Hz,1H),5.30(brs,1H),4.95‐4.91(m,1H),4.52‐4.28(m,3H),3.95‐3.65(m,6H),3.25‐ 3.08(m,2H),2.82(s,4H),2.32(s,3H),1.44‐1.35(m,6H),1.18(d,J=6.0Hz,6H).
Embodiment 3
[0099] Example 3. Preparation of (2R, 3R, 4R, 5R)-5-(2,4-diketone-3,4-dihydropyrimidine-1(2H)-position)-4-fluoro-2-((( (S)‐(((S)‐1‐isopropoxy‐1‐ketopropane‐2‐position)amino)(phenoxy)phosphoryl)oxy)methyl)‐4‐methyltetrahydrofuran‐3‐ position 3-morpholin
[0100] Ester (I‐3)
[0101]
[0102] According to the synthesis method of compound I-1, compound I-3 can be prepared by replacing 2-morpholine acetic acid with 3-morpholine acetic acid.
[0103] 1 H‐NMR (400MHz, CDCl 3 )δ: 8.43(s, 1H), 7.57(d, J=8.0Hz, 1H), 7.36(t, J=8.0Hz, 2H), 7.26‐7.23(m, 2H), 7.20(t, J=7.6 Hz,1H),6.24(d,J=18.8Hz,1H),5.54(d,J=8.4Hz,1H),5.28(dd,J 1 =9.2Hz,J 2 =20.8Hz,1H),5.05‐4.99(m,1H),4.60‐4.55(m,1H),4.34(d,J=9.6Hz,1H),4.30‐4.25(m,1H),4.03‐3.95( m,1H),3.87(t,J=6.4Hz,1H),3.69‐3.67(m,4H),2.74‐2.70(m,2H),2.65‐2.62(m,2H),2.48(br,4H) ,1.40‐1.37(m,6H),1.26(d,J=6.4Hz,6H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com