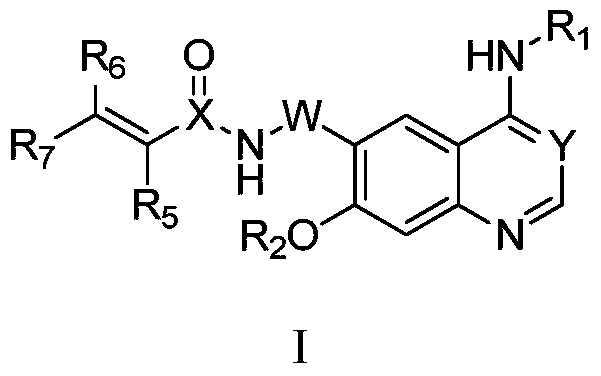
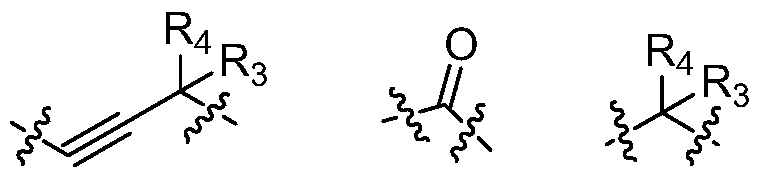
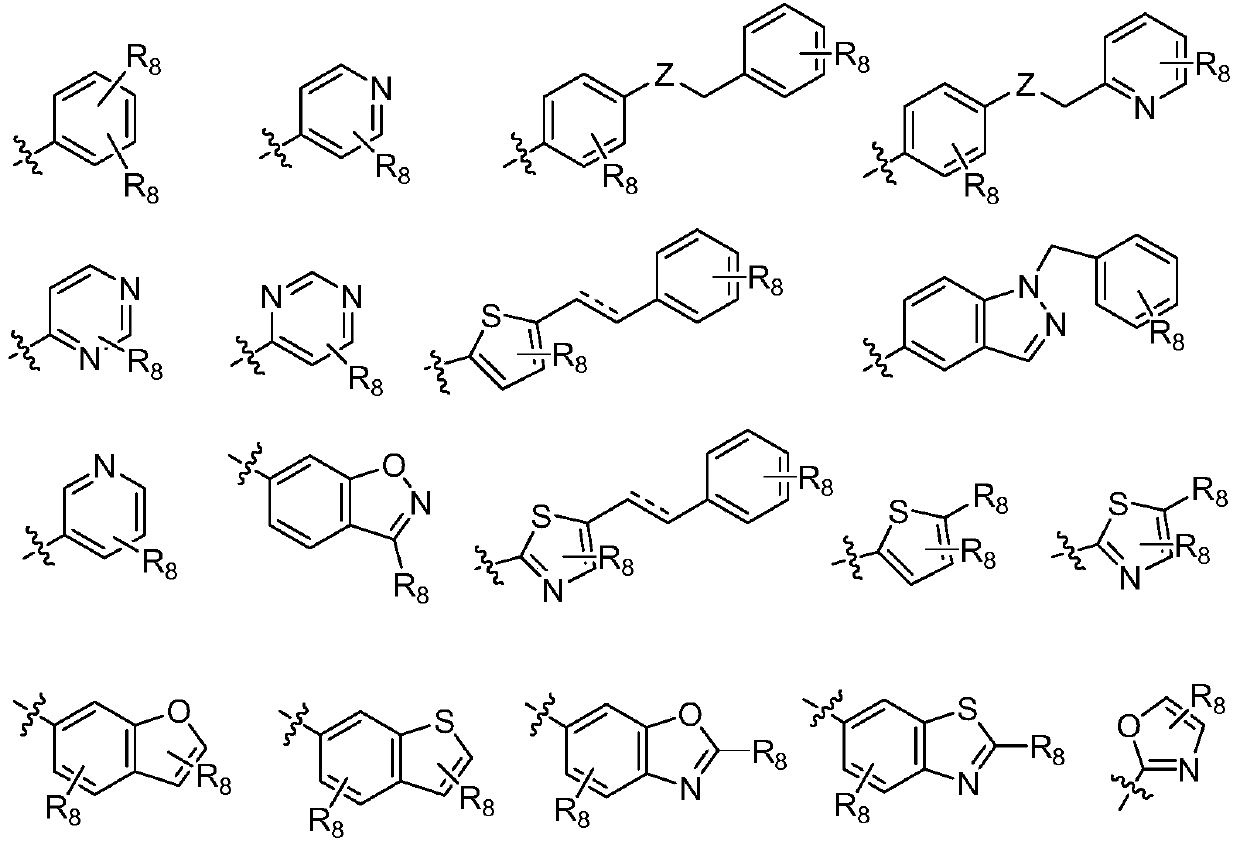
Quinazoline derivatives and their preparation methods and applications
A technology of quinazoline and derivatives, applied in the field of quinazoline derivatives and their preparation, can solve the problems of loss of drug efficacy, increase of EGFR and ATP binding ability and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0106] Compound 7-1, N-(4-(4-((3-Chloro-4-fluorophenyl)amino)-7-(2-(dimethylamino)ethoxy)quinazolin-6-yl) - Preparation of 2-methylbutyl-3-yn-2-yl)acrylamide.
[0107] Synthesize as follows:
[0108]
[0109] Step (1) prepares compound 2 (that is, methyl 2-amino-4-fluoro-5-iodobenzoate) as follows:
[0110] Take a 250mL clean two-necked round-bottomed flask, add 50g of raw material 1 (322.58mmol), 100mL of tert-butanol and 50mL of water in turn, add 44.g (161.29mmol) of elemental iodine in batches under stirring, and then slowly dropwise add 40mL of 30% hydrogen peroxide , heated to 50°C in an oil bath for 2h. The reaction was monitored by TLC. After the reaction was completed, the system was cooled to room temperature, 50 mL of saturated aqueous sodium bisulfite solution was added, and then extracted three times with 150 mL of ethyl acetate. The organic phases were combined, washed with 50 mL of saturated brine, dried over anhydrous sodium sulfate, and recrystallized to...
Embodiment 2
[0123] Compound 7-2, N-(3-(4-((3-chloro-4-fluorophenyl)amino)-7-(2-morpholinoethoxy)quinazolin-6-yl)propyl - Preparation of 2-yn-1-yl)acrylamides.
[0124] Referring to the preparation method of Example 1, N-(2-hydroxyethyl)morpholine is selected as the raw material in step (3), and the operation method is as in Example 1. The target compound 7-2.
[0125]
[0126] The characterization data for this target compound are: 1 H NMR (400MHz, DMSO-d 6 )δ9.84(s,1H),8.69(t,J=5.6Hz,1H),8.63(s,1H),8.56(s,1H),8.19(dd,J=6.9,2.6Hz,1H), 7.82(ddd,J=9.0,4.3,2.7Hz,1H),7.42(t,J=9.1Hz,1H),7.23(s,1H),6.27(dd,J=17.1,10.0Hz,1H),6.16 (dd, J=17.1, 2.3Hz, 1H), 5.66 (dd, J=10.0, 2.4Hz, 1H), 4.26-4.31 (m, 4H), 3.60 (q, J=4.7Hz, 4H), 2.79- 2.83(m,2H),2.57-2.62(m,4H).
Embodiment 3
[0128] Compound 7-3, N-(3-(4-((3-chloro-4-fluorophenyl)amino)-7-(2-(dimethylamino)ethoxy)quinazolin-6-yl) Preparation of propyl-2-yn-1-yl)acrylamide.
[0129] According to the method of Example 1, the target compound 7-3 is prepared by using compound 6-2 as a raw material in step 5).
[0130]
[0131] The characterization data for this target compound are: 1 H NMR (400MHz, DMSO-d 6 )δ9.93(s,1H),8.78(t,J=5.8Hz,1H),8.69(s,1H),8.56(s,1H),8.21(dd,J=7.0,2.6Hz,1H), 7.84(dt,J=7.5,3.4Hz,1H),
[0132] 7.42(t, J=9.1Hz, 1H), 7.22(s, 1H), 6.29(dd, J=17.1, 10.1Hz, 1H), 6.16(d, J=16.9Hz, 1H),
[0133] 5.66(dd, J=10.0, 2.4Hz, 1H), 4.21-4.29(m, 4H), 2.77(t, J=5.4Hz, 2H), 2.31(s, 6H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com