Tyrosine kinase irreversible inhibitor and preparation method and applications thereof
A tyrosine kinase and inhibitor technology, applied in the field of tyrosine kinase irreversible inhibitors or their pharmaceutically acceptable salts and their preparation, can solve problems such as loss of activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
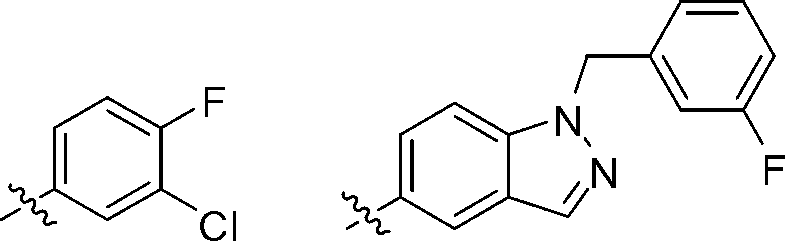
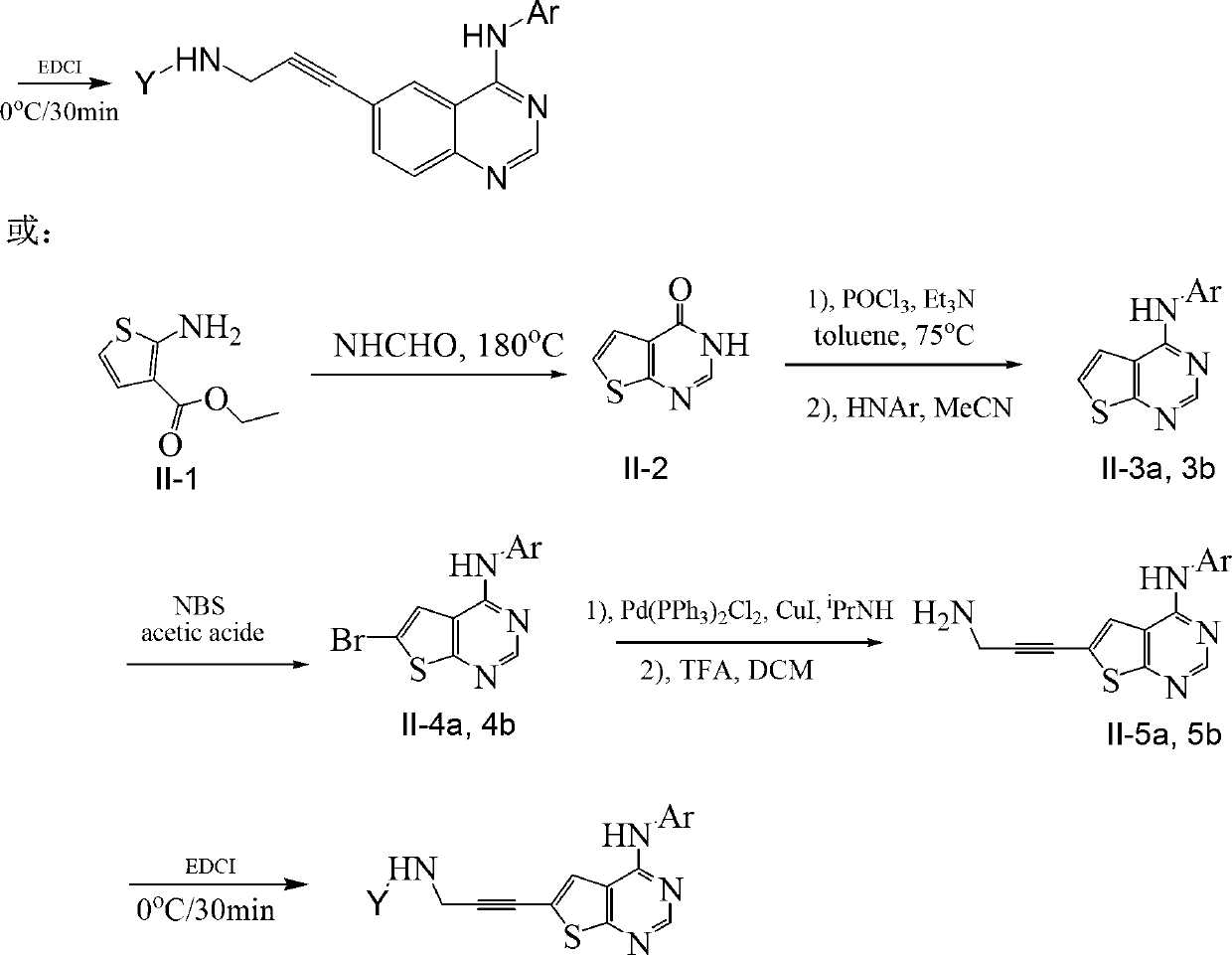
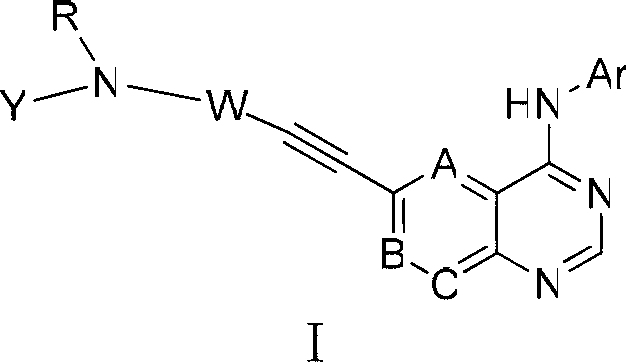
[0074] Compound 1, N-(3-(4-(1-(3-fluorophenyl)-1H-indazol-5-amino)-quinazolin-6-yl)-prop-2-yne)-acrylamide preparation.
[0075] Line one:
[0076]
[0077] According to above-mentioned route, step 1) prepares compound I-2, i.e. 2-amino-5-iodobenzoic acid methyl ester, method is as follows:
[0078] In a 250mL two-necked flask equipped with a dropping funnel, add 50.19g (332.42mmol) methyl anthranilate, 100mL tert-butyl alcohol and 50mL water, add 44.32g (174.52mmol) elemental iodine in batches under stirring, then slowly Add 40mL of 30% hydrogen peroxide dropwise, and heat to 50°C in an oil bath for 2h. The reaction was monitored by TLC. After the reaction, cool the reaction system to room temperature, add 50 mL of saturated aqueous sodium bisulfite solution, stir well, then extract three times with 150 mL of ethyl acetate, combine the organic phases, wash with 50 mL of saturated brine, dry over anhydrous sodium sulfate, and spin evaporate About 100 mL of ethyl acetate...
Embodiment 2
[0103] Compound 2, (E)-N-(3-(4-(1-(3-fluorophenyl)-1H-indazol-5-amino)-quinazolin-6-yl)-prop-2- Preparation of alkyne)-but-2-enamide.
[0104] Step 1)-4) is the same as embodiment 1, and step 5) is as follows:
[0105] Select 2-butenoic acid to perform condensation reaction with 1-6a, and the operation method is as in Example 1 to obtain the target product.
[0106] The characterization data of this compound are: 1H NMR (400MHz, DMSO-d6) δppm 10.01(s, 1H), 8.50(s, 1H), 8.25(s, 1H), 8.16(s, 1H), 7.84(s, 1H ), 7.72(s, 1H), 7.54-7.64(m, 3H), 7.33-7.39(m, 1H), 7.04-7.12(m, 4H), 6.67-6.72(m, 1H), 5.95(d, J =14.8Hz, 1H), 5.70(s, 2H), 4.27(d, J=5.2Hz, 2H), 1.81(d, J=6.0Hz, 3H).ESI-MS m / z: 491.2(M+H ), 489.2 (M-H).
[0107] The compound structural formula is:
[0108]
Embodiment 3
[0110] Compound 3, (E)-N-(3-(4-(1-(3-fluorophenyl)-1H-indazol-5-amino)-quinazolin-6-yl)-prop-2- Preparation of alkyne)-4-morpholinobutyl-2-enamide.
[0111] Step 1)-4) is the same as embodiment 1, and step 5) is as follows:
[0112] 4-Morpholine 2-butenoic acid was selected for condensation reaction with 1-6a, and the operation method was as in Example 1 to obtain the target product.
[0113] The characterization data of this compound are: 1H NMR (400MHz, DMSO-d6) δppm 10.01(s, 1H), 8.50(s, 1H), 8.25(s, 1H), 8.16(s, 1H), 7.84(s, 1H ), 7.72(s, 1H), 7.54-7.64(m, 3H), 7.33-7.39(m, 1H), 7.04-7.12(m, 4H), 6.67-6.72(m, 1H), 6.05-6.15(m , 1H), 5.70(s, 2H), 4.27(d, J=4.0Hz, 2H), 3.53(t, J=4.8Hz, 4H), 3.04(d, J=6.4Hz, 2H), 2.32(s , 4H). ESI-MS m / z: 576.2 (M+H), 574.2 (M-H).
[0114] The compound structural formula is:
[0115]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com