A Synthetic Method of Mesoporous γ-al2o3 with Excellent Performance
A synthesis method and mesoporous technology, applied in the field of preparing mesoporous γ-Al2O3, can solve the problems of small pore volume, complex operation steps, mesoporous, high cost, etc., achieve high mechanical strength, short reaction time, avoid alkyl The effect of the use of aluminum
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
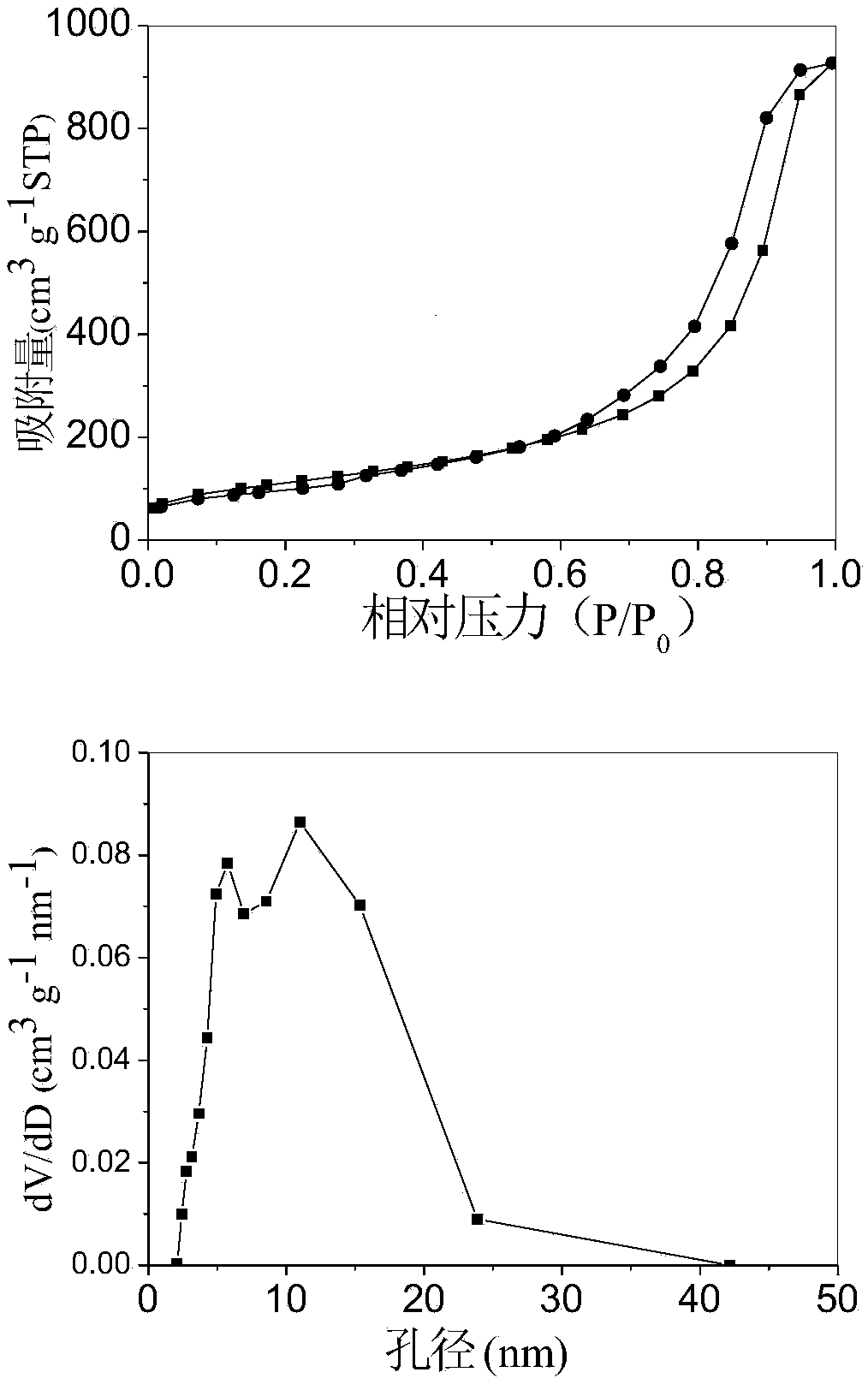
[0029] At room temperature, will contain NaAlO 2 solution with AlCl 3 ·6H 2 O solution, according to AlO 2 - and Al 3+ A molar ratio of 2.78 was mixed cocurrently to produce a white precipitate, and the resulting mixture was stirred at room temperature for 2 hours. The mixture was suction filtered and washed to obtain aluminum hydroxide precipitate, which was redispersed in Al 3+ The P123 solution with a molar ratio of 29 / P123 was mechanically stirred for 2 hours. The uniformly stirred composite was dried in an oven at 100°C for 12 hours, and the dried composite was transferred to a muffle furnace, heated to 550°C at a heating rate of 2°C / min, and then roasted at a constant temperature of 550°C for 2 hours. Depend on figure 1 N in 2 The adsorption-desorption isotherm and pore size distribution curve show that the γ-Al 2 o 3 is a mesoporous material, the resulting mesoporous γ-Al 2 o 3 The specific surface area is 400.9m 2 / g, the pore volume is 1.43cm 3 / g, the p...
Embodiment 2
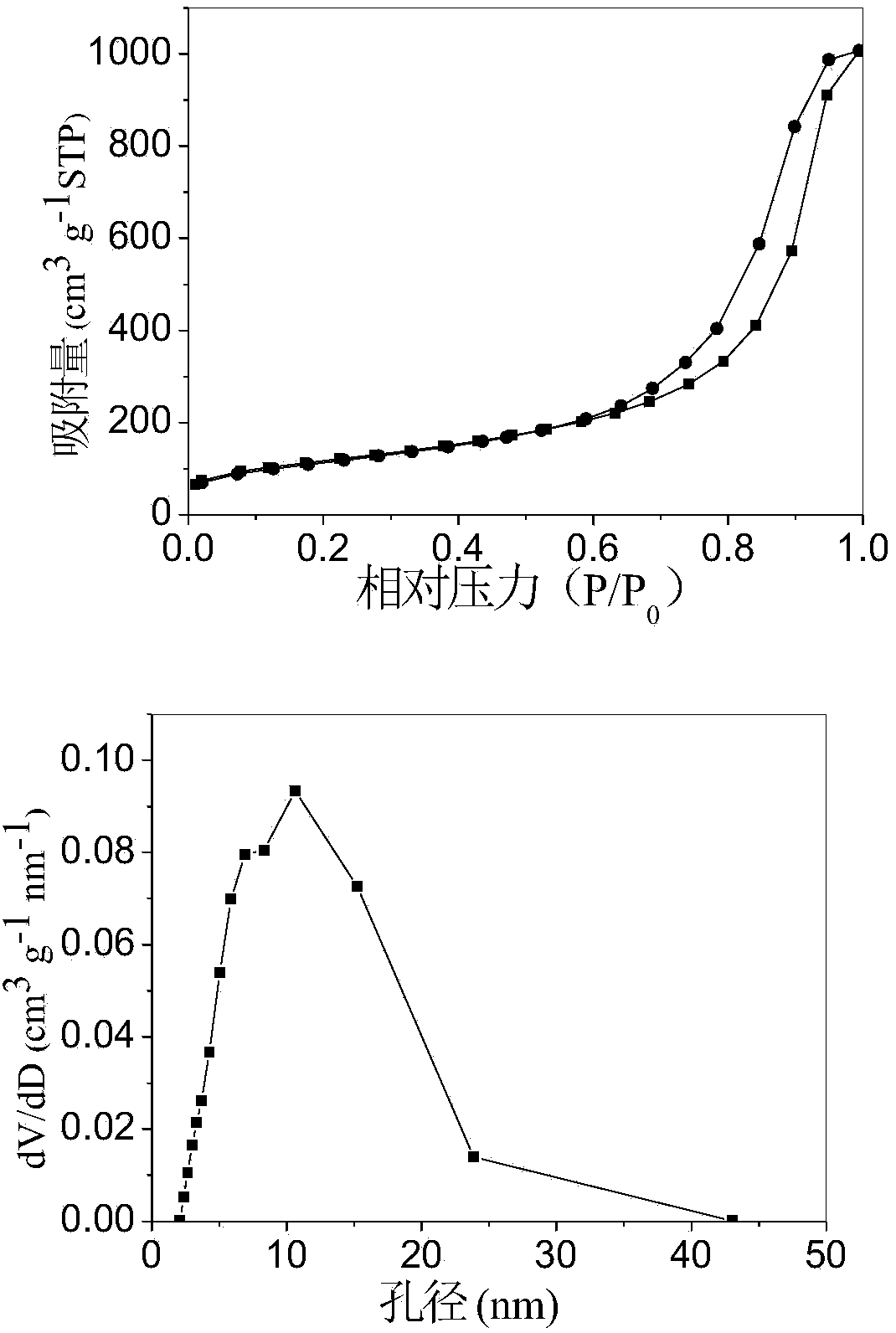
[0031] At room temperature, will contain NaAlO 2 solution with AlCl 3 ·6H 2 O solution, according to AlO 2 - and Al 3+ A molar ratio of 2.78 was mixed cocurrently to produce a white precipitate, and the resulting mixture was stirred at room temperature for 2 hours. The mixture was suction filtered and washed to obtain aluminum hydroxide precipitate, which was redispersed in Al 3+ The P123 solution with a molar ratio of / P123 of 22.7 was mechanically stirred for 2 hours. The uniformly stirred composite was dried in an oven at 100°C for 12 hours, and the dried composite was transferred to a muffle furnace, heated to 550°C at a heating rate of 2°C / min, and then roasted at a constant temperature of 550°C for 2 hours. Depend on image 3 N in 2 The adsorption-desorption isotherm and pore size distribution curve show that the γ-Al 2 o 3 is a mesoporous material, the resulting mesoporous γ-Al 2 o 3 The specific surface area is 421.9m 2 / g, the pore volume is 1.56cm 3 / g,...
Embodiment 3
[0033] At room temperature, will contain NaAlO 2 solution with AlCl 3 ·6H 2 O solution, according to AlO 2 - and Al 3+ A molar ratio of 2.56 was mixed cocurrently to produce a white precipitate, and the resulting mixture was stirred at room temperature for 2 hours. The mixture was suction filtered and washed to obtain aluminum hydroxide precipitate, which was redispersed in Al 3+ The P123 solution with a molar ratio of 38.7 / P123 was mechanically stirred for 2 hours. The uniformly stirred composite was dried in an oven at 100°C for 12 hours, and the dried composite was transferred to a muffle furnace, heated to 550°C at a heating rate of 2°C / min, and then roasted at a constant temperature of 550°C for 2 hours. Depend on Figure 5 N in 2 The adsorption-desorption isotherm and pore size distribution curve show that the γ-Al 2 o 3 is a mesoporous material, the resulting mesoporous γ-Al 2 o 3 The specific surface area is 400.3m 2 / g, the pore volume is 1.38cm 3 / g, th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com