Modified benzoxazine resin and composition application thereof
A resin composition, benzoxazine technology, applied in electrical components, organic chemistry, printed circuits, etc., can solve the problems of low glass transition temperature, high glass transition temperature, unsatisfactory, etc., and achieve the effect of low dielectric loss
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
manufacture example
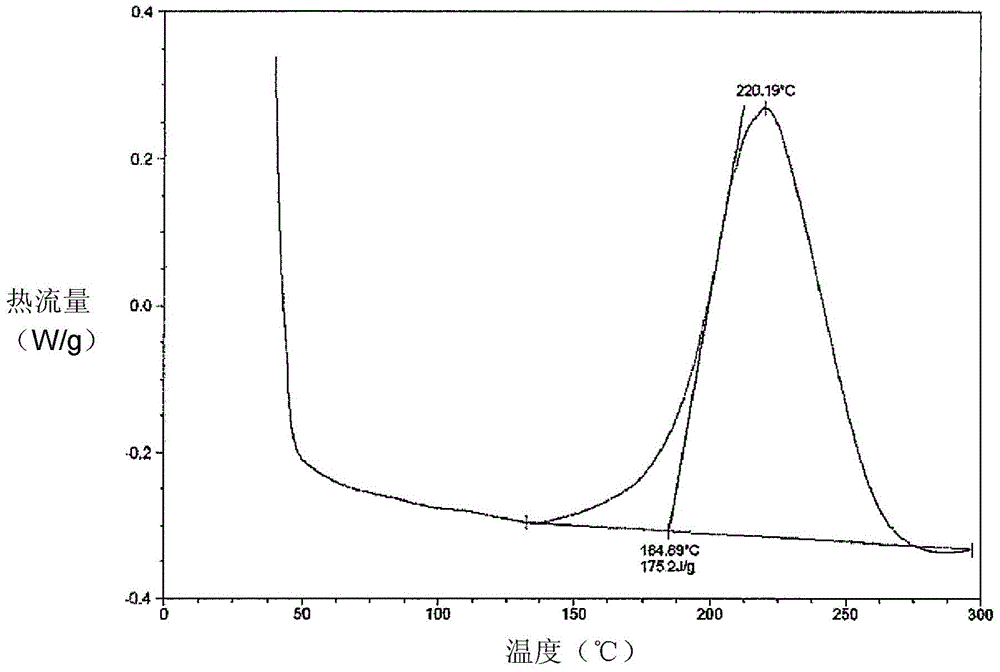
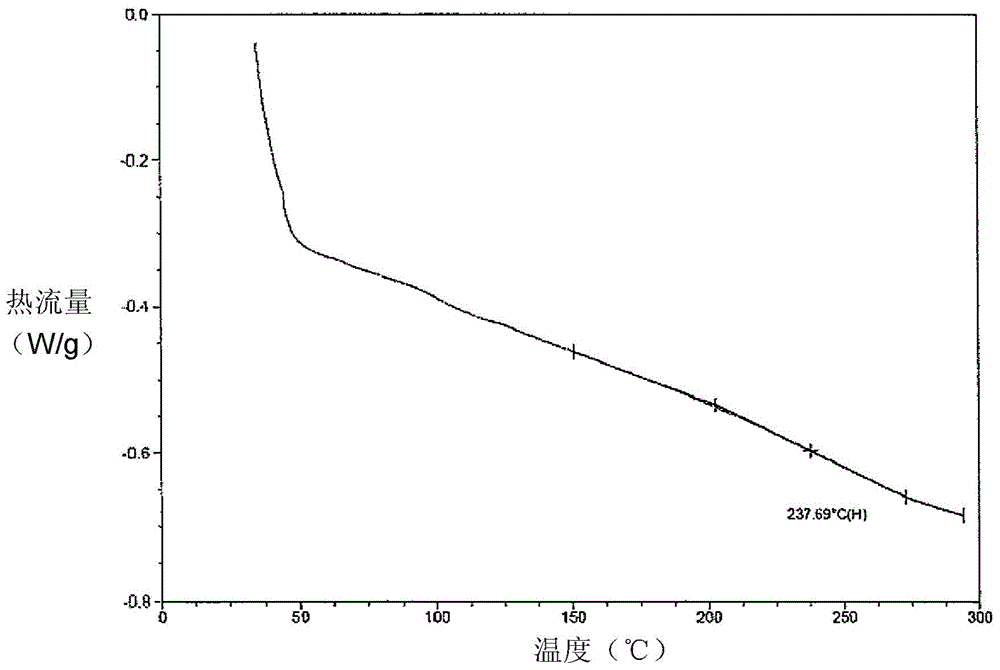
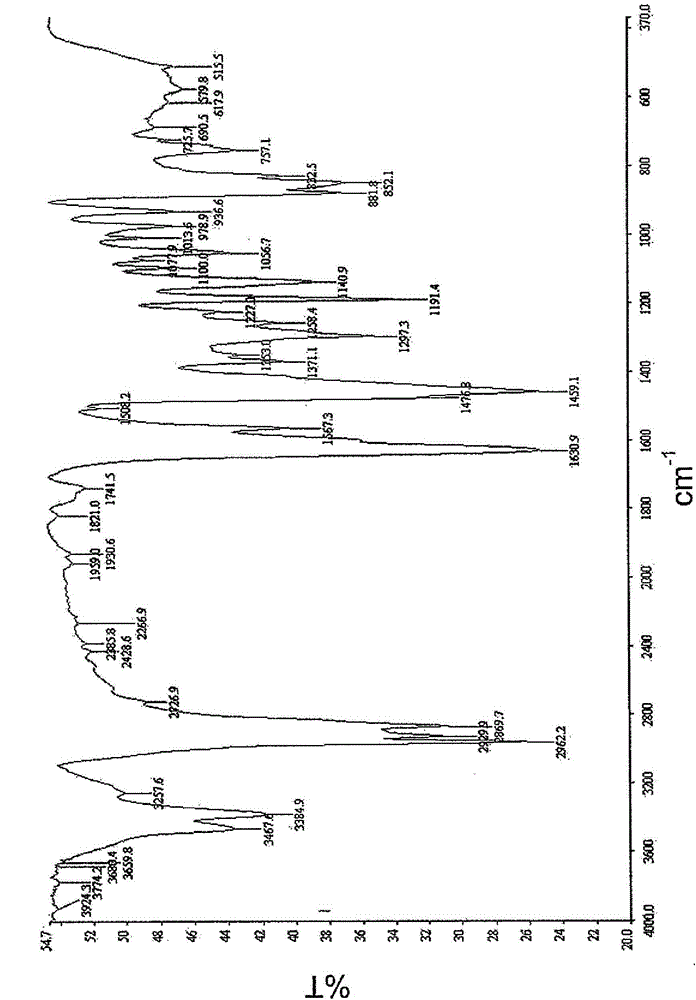
[0070] Set up 3 liters of reaction vessels, which are equipped with reflux condensers, thermometers, and stirring devices. 134.0 grams (1mol) of terephthalaldehyde, 218 grams (2mol) of 4-aminophenol, 205.7 grams of propylene glycol monomethyl ether and 178.0 grams of Toluene was added for blending, stirred and heated, followed by reflux dehydration for 4 hours, and cooled to room temperature at about 115 to 125° C. to obtain a polyazomethine compound (Compound A). 388g of compound A was added to a 3-liter glass-jacketed reactor, followed by 172g of formaldehyde, 242ml of xylene and 484ml of butanol. The reaction mixture was heated to 80°C to 82°C with constant stirring to mix. Finally, 238 g of aniline was added, heated to 90 to 95° C., and refluxed for 6 hours. Add another 600ml xylene and 1200ml butanol in the final reaction mixture, so that the reaction temperature is reduced to normal temperature, the alcohol solvent is removed, and the modified benzoxazine compound product...
Embodiment
[0075] According to the formula listed in Table 1, the relevant components are fully mixed to obtain the resin varnish of the resin composition, wherein E1 to E8 represent the embodiments of the resin composition of the present invention, and C1 to C2 represent the comparative examples of the aforementioned resin composition. It should be noted that although the present invention distinguishes the composition into examples and comparative examples in order to demonstrate the efficacy of certain components or dosages, this distinction is only for convenience of description and does not mean that the comparative examples do not belong to a part of the present invention .
[0076] The names of chemicals used in the following examples and comparative examples are as follows:
[0077] LZ 8280: bisphenol F type benzoxazine resin (BPF-Bz), available from Huntsman;
[0078] LZ 8290: bisphenol A type benzoxazine resin (BPA-Bz), available from Huntsman;
[0079] LZ 8270: phenolphthale...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com