Method for removing metal ions in graphite oxide
A technology of metal ions and graphite, which is applied in the field of removal of metal ions in graphite oxide, can solve the problems of weakened electrostatic force of graphene oxide, difficult to be washed away, and edge defects of graphene, so as to reduce the amount of sewage treatment and increase static electricity. function, the effect of improving the removal efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] The graphite oxide used in this example is obtained by the following preparation method: in an ice-water bath at 0°C to 15°C, add 1 g of graphite into 100 mL of concentrated sulfuric acid with a mass concentration of 95% to 98%, and divide it within 1 hour under stirring. A total of 3g of potassium permanganate was added for 6 times. After the addition, continue to stir in the ice-water bath for 1h, then raise the temperature to 35°C, keep it for 2h, add 215mL of deionized water, then raise the temperature to 98°C, keep it for 1h, and then Add 200 mL of deionized water and 30% hydrogen peroxide until the solution has no bubbles, and after suction filtration is completed, graphite oxide is obtained.
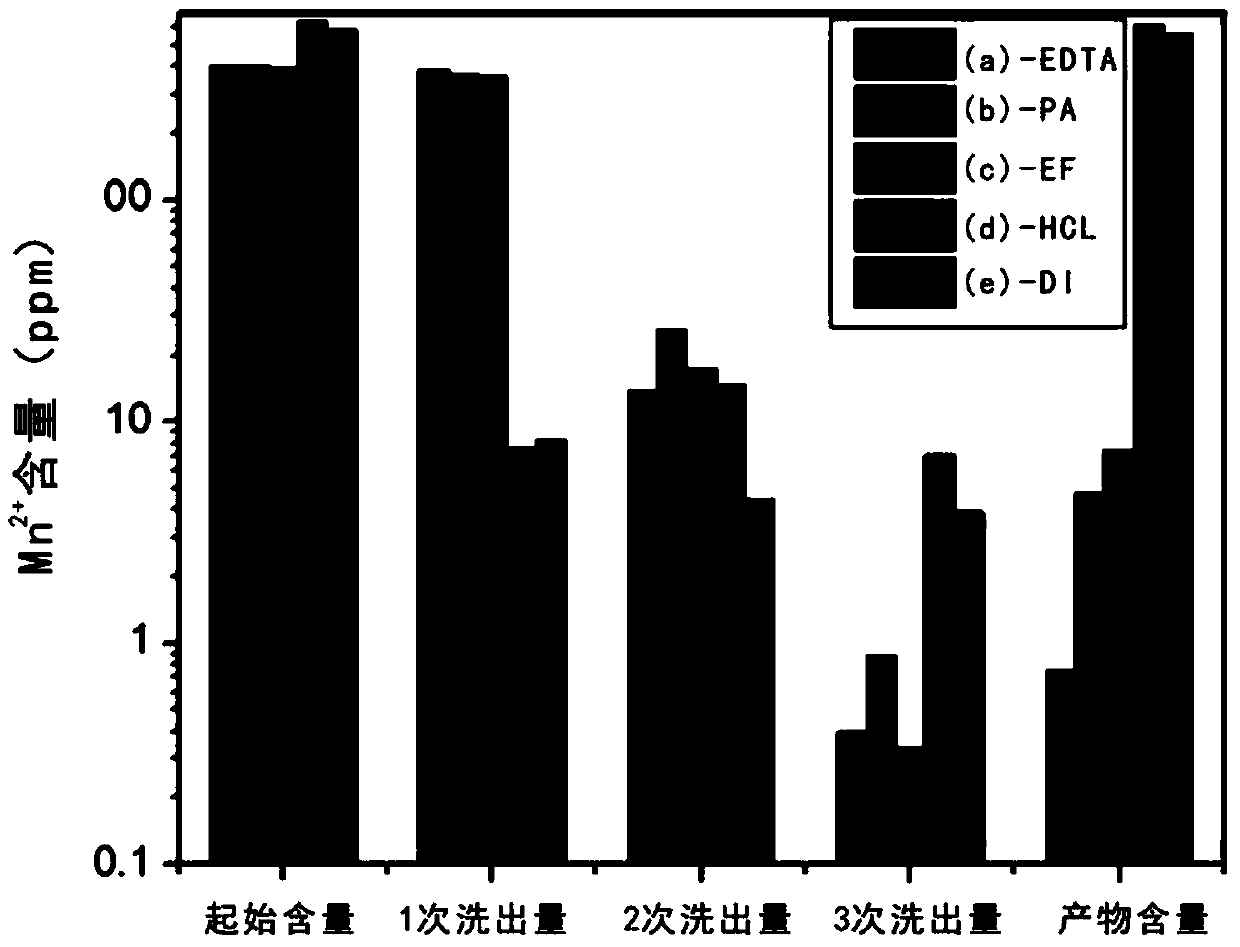
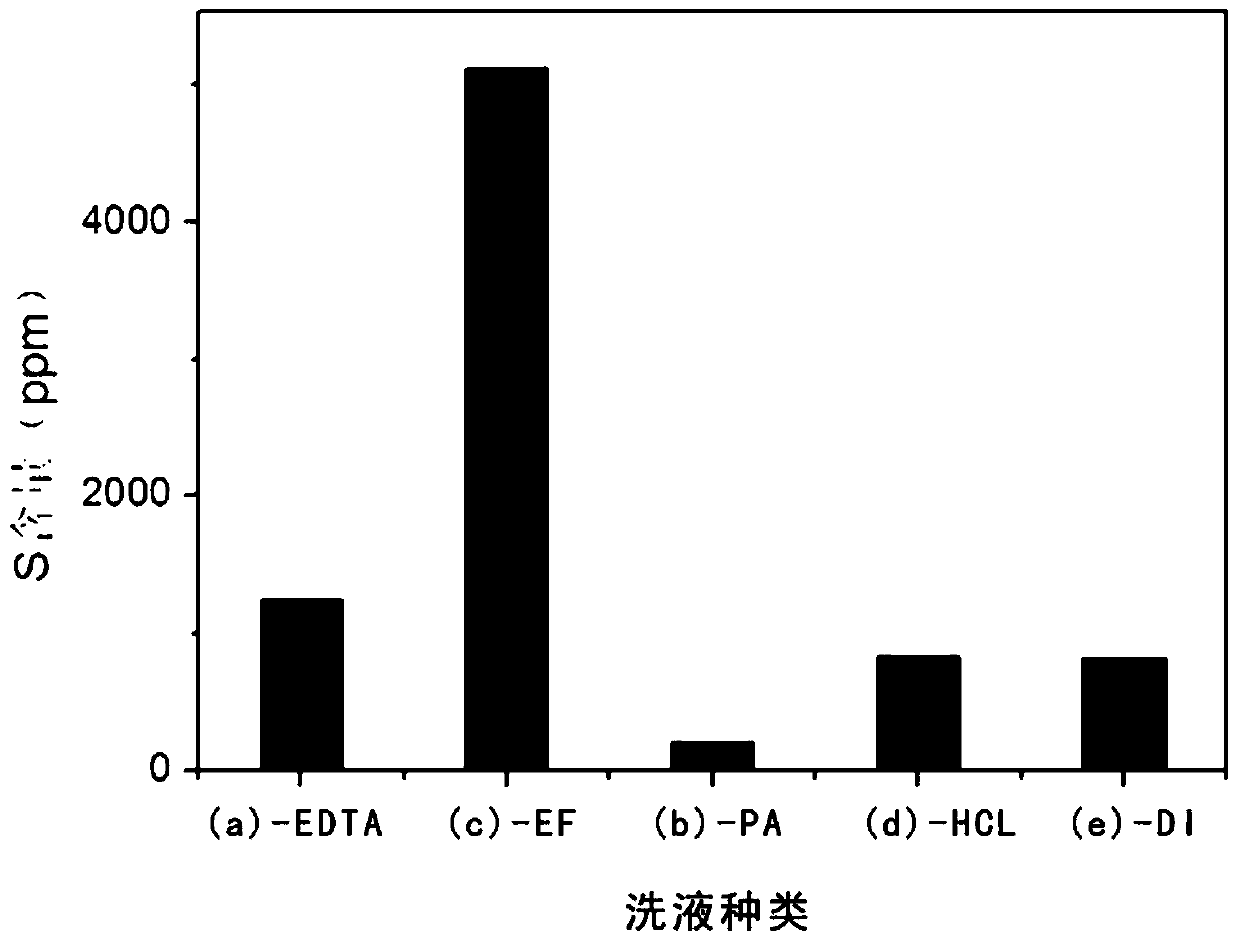
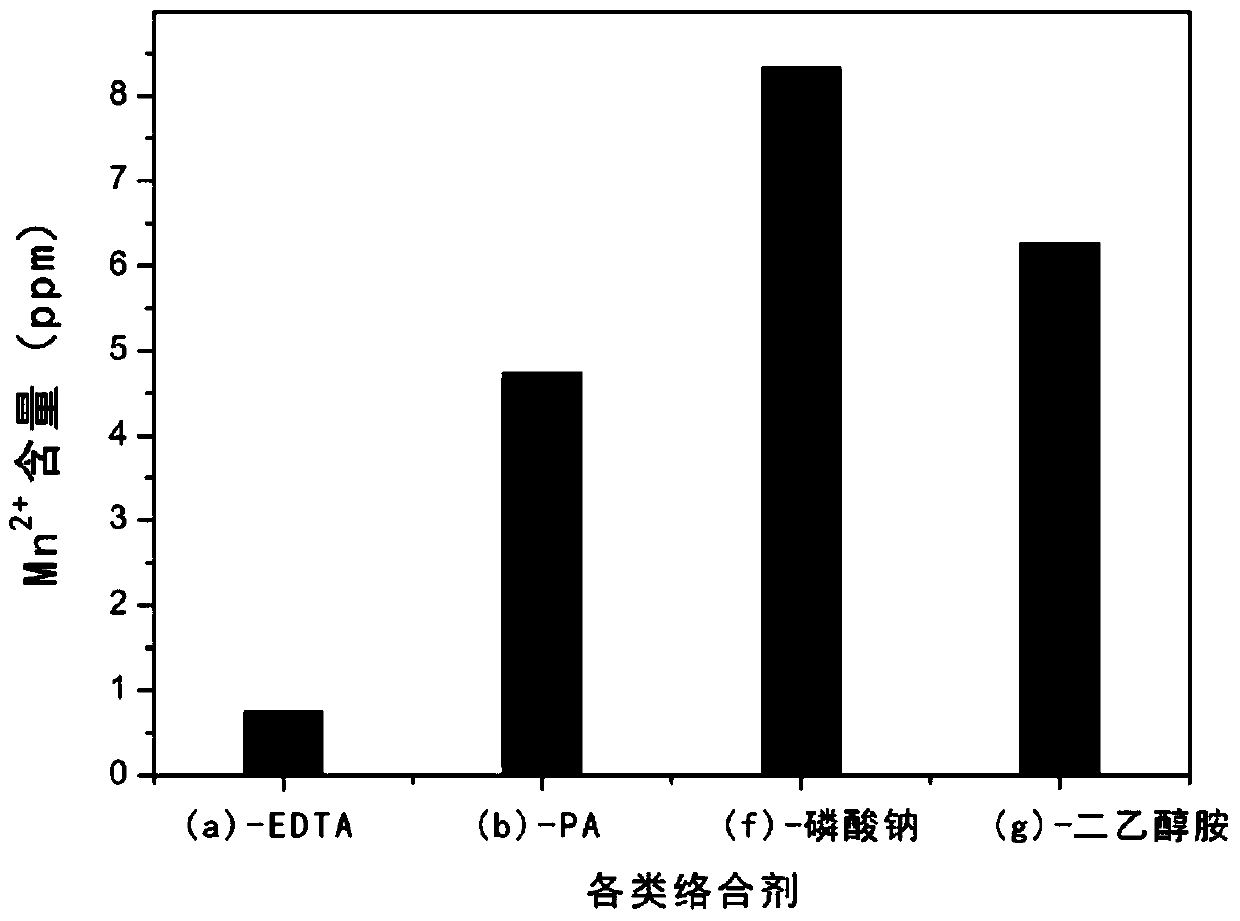
[0030] First use hydrochloric acid and deionized water to prepare an acidic aqueous solution with a pH value of 3, then add disodium EDTA to the acidic aqueous solution at a mass ratio of 1:200 with deionized water and stir evenly; get the obtained graphite oxide, press each...
Embodiment 2
[0033] The graphite oxide preparation method that present embodiment adopts is identical with embodiment 1, omission.
[0034] First, use phytic acid and deionized water at a mass ratio of 1:500 to prepare an acidic aqueous solution with a pH of 1.6; take the obtained graphite oxide, take 800mL of the prepared acidic aqueous solution each time, and centrifuge at a speed of 3000r / min Remove the supernatant for 10 minutes, and repeat the washing for a total of three times; then use 800 mL of deionized water, centrifuge and wash for 10 minutes at a speed of 3000 r / min, after removing the supernatant, add the obtained graphite oxide to 800 mL of deionized water to disperse and freeze Drying yielded graphite oxide solid.
[0035] According to the ICP test, the manganese ion content in the graphite oxide solid is 4.7449ppm.
Embodiment 3
[0037] The graphite oxide preparation method that present embodiment adopts is identical with embodiment 1, omission.
[0038] First use nitric acid and deionized water to prepare an acidic aqueous solution with a pH value of 3, then add sodium phosphate to the pickling aqueous solution at a mass ratio of 1:500 to deionized water and stir evenly; take the obtained graphite oxide, press each Take the prepared 800mL acidic aqueous solution, centrifuge and wash at 3000r / min for 10min to remove the supernatant, repeat the washing for a total of three times, then use 800mL of deionized water, centrifuge and wash at 3000r / min for 10min, remove the supernatant After clearing the liquid, the obtained graphite oxide was added to 800 ml of deionized water to disperse and freeze-dried to obtain graphite oxide solid.
[0039] According to the ICP test, the manganese ion content in the graphite oxide solid is 8.326ppm.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com