A kind of acetylhydrazone compound and preparation method thereof
A technology of compound and acetyl hydrazone, which is applied in the field of acetyl hydrazone compounds and its preparation, can solve the problems of in-depth research on compounds with antimicrobial activity, decreased drug treatment effect, and increased drug resistance, so as to solve bacterial drug resistance, The effect of low cost and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
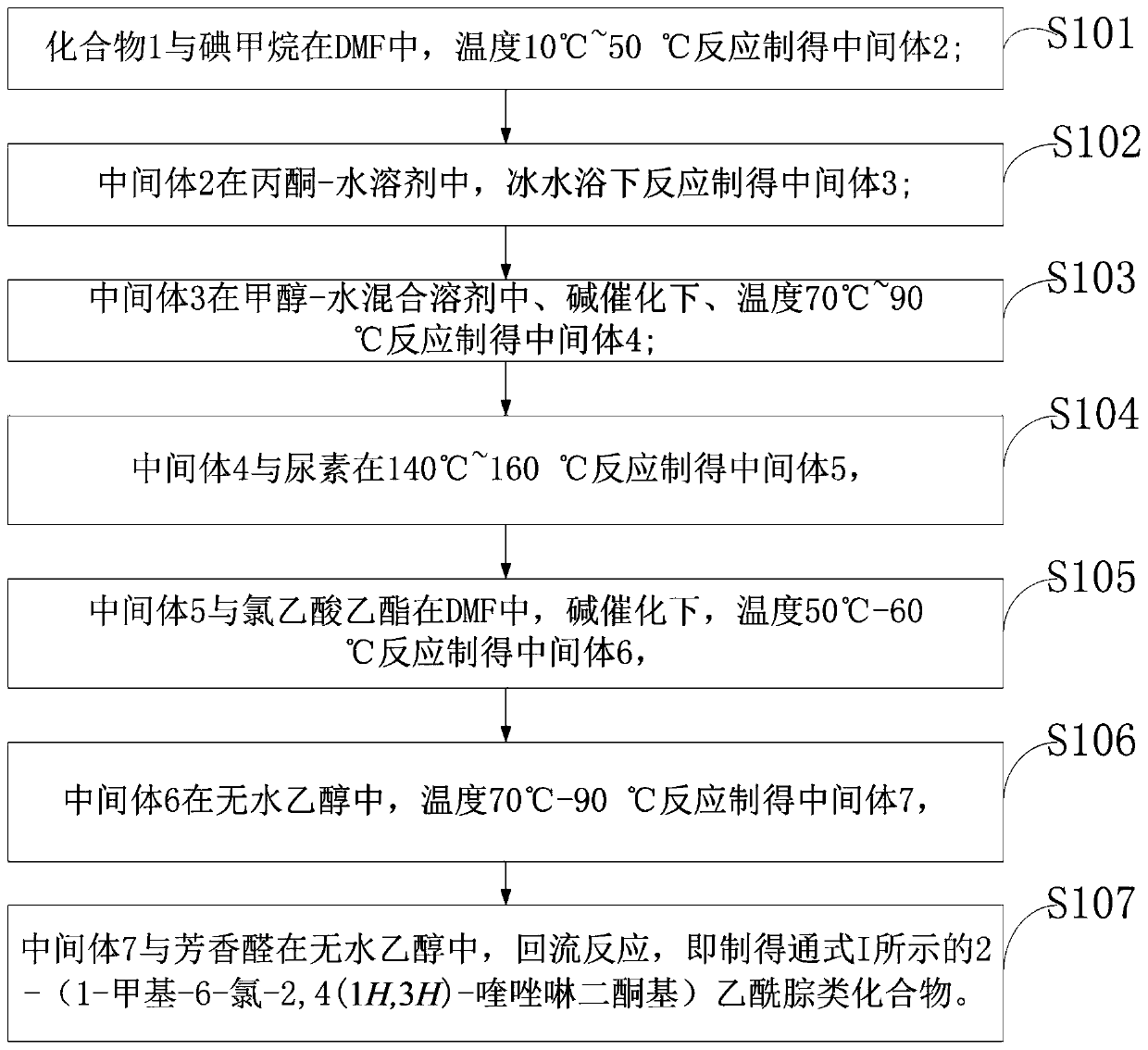
[0058] Such as figure 1 Shown: the preparation method of the acetylhydrazone compound of the embodiment of the present invention specifically comprises the following steps:
[0059] S101: Compound 1 reacts with methyl iodide in DMF at a temperature of 10°C to 50°C to prepare intermediate 2;
[0060] S102: intermediate 2 is reacted in an acetone-water solvent under an ice-water bath to obtain intermediate 3;
[0061] S103: intermediate 3 is reacted in a methanol-water mixed solvent under alkali catalysis at a temperature of 70°C to 90°C to prepare intermediate 4;
[0062] S104: intermediate 4 is reacted with urea at 140°C to 160°C to prepare intermediate 5,
[0063] S105: intermediate 5 is reacted with ethyl chloroacetate in DMF under alkali catalysis at a temperature of 50°C-60°C to prepare intermediate 6,
[0064] S106: intermediate 6 is reacted in absolute ethanol at a temperature of 70°C-90°C to prepare intermediate 7,
[0065] S107: Intermediate 7 and aromatic aldehyde...
Embodiment 1
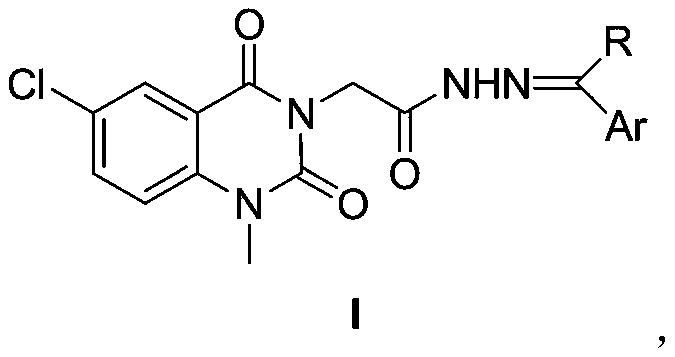
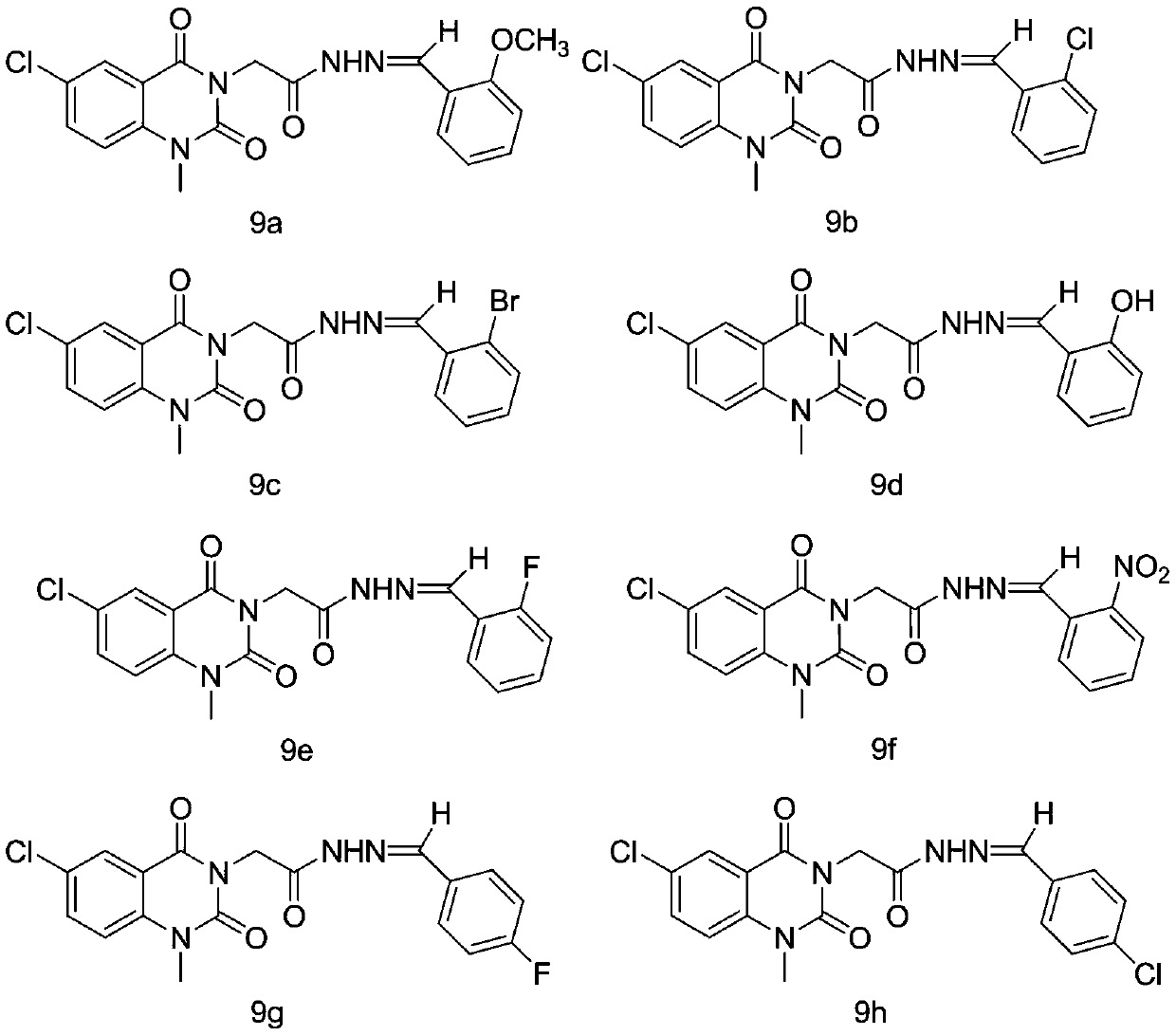
[0072] Embodiment 1, preparation of 2-(1-methyl-6-chloro-2,4(1H,3H)-quinazolinedionyl) acetylhydrazone compounds shown in general formula I
[0073] 1, the preparation of intermediate 2 (methyl o-thaminobenzoate)
[0074]
[0075] In a 500mL flask with a drying tube, add 50.20g (0.33mol) of raw material 1 (methyl anthranilate), 26.58g (0.66mol) of sodium hydroxide, 100mL of dimethylformamide (DMF), and drop Add a mixture of 61.32g (0.43mol) of iodomethane and 100mL of DMF, add and react at room temperature for 1 hour, then heat to 45°C and react for 7 hours. After the reaction, cool to room temperature, add 200mL of water, and extract with ethyl acetate (5× 100 mL), combined the ethyl acetate extracts, dried over anhydrous magnesium sulfate, suction filtered, the filtrate was rotary evaporated to remove the solvent, and purified by column to obtain 29.50 g of intermediate 2 (light yellow liquid), with a yield of 54%.
[0076] 2. Preparation of intermediate 3 (2-amino-5-chl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com