A formate dehydrogenase mutant with improved enzyme activity and stability and its construction method
A technology for formate dehydrogenase and mutants, applied in the field of genetic engineering, can solve the problems of low specific enzyme activity and poor operational stability, and achieve the effects of improving acid resistance, eliminating resistance, and increasing specific enzyme activity and catalytic efficiency.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1 Construction of recombinant vector containing formate dehydrogenase mutant
[0029] (1) Obtaining of A10C mutant: Using the nucleotide sequence shown in SEQ ID NO.4 as a template, Fprimer (sequence shown in SEQ ID NO.5) and Rprimer (sequence shown in SEQ ID NO.6) are Primer, PCR is performed to obtain the recombinant gene shown in SEQ ID NO.3.
[0030] (2) Combine the recombinant gene with pET28a They were digested with EcoR I and Xho I. After purification, they were ligated with T4 DNA ligase at 16°C overnight. The ligation product was chemically transformed into E.coli BL21 competent cells. The transformation solution was coated on an LB plate containing kanamycin (50 mg / L), the plasmid was extracted, and the recombinant plasmid constructed by double enzyme digestion was verified and named pET28a-A10C. The sequencing work was completed by Shanghai Shenggong.
Embodiment 2
[0031] Example 2 Construction of recombinant Escherichia coli engineering bacteria producing formate dehydrogenase mutants
[0032] The strain containing the correct recombinant plasmid pET28a-A10C obtained in Example 1 is the recombinant genetically engineered strain pET28a-A10C / E.coli BL21.
Embodiment 3
[0033] Example 3 Recombinant strain pET28a-A10C / E.coli BL21 expresses formate dehydrogenase and determination of enzyme activity
[0034] The recombinant strain pET28a-A10C / E.coli BL21 constructed in Example 2 and the control strain pET28a-FDH / E.coli BL21 expressing the unmutated wild enzyme CboFDH (amino acid sequence shown in SEQ ID NO: 2) were respectively inoculated into 10 mL of kanamycin-containing LB medium, shake culture overnight at 37°C, transfer to TY fermentation medium at a 4% inoculum volume the next day, culture at 37°C for 4 hours, add 0.5mM IPTG to induce at 24°C 16 hour. The cells were collected by centrifugation and crushed, and the supernatant of cell crushing (crude enzyme solution) was used for the determination of enzyme activity.
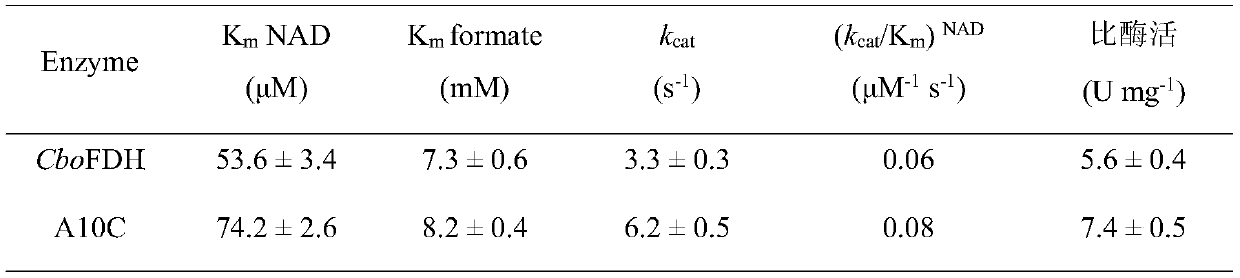
[0035] The obtained crude enzyme solution was purified to obtain formate dehydrogenase mutant A10C, and the kinetic parameters of the purified recombinant formate dehydrogenase mutant A10C were analyzed, as shown in Table 1. Muta...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com