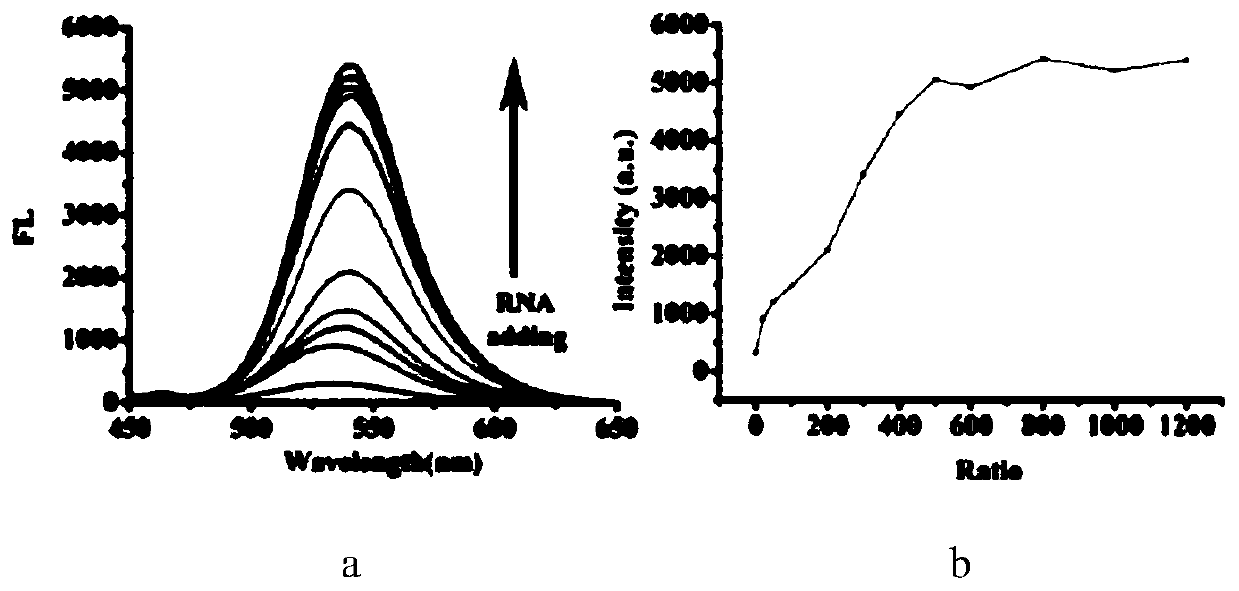
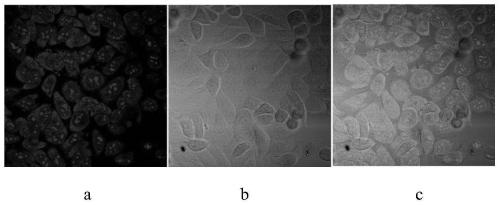
A two-photon fluorescent probe for RNA imaging and its application in living cells
A two-photon fluorescence and imaging technology, applied in the field of fluorescent probes, can solve the lack of two-photon fluorescent probes and other problems, and achieve the effects of good membrane permeability, good photostability, and strong color rendering
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
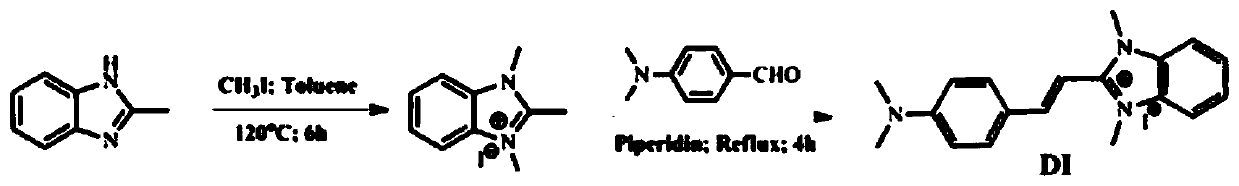
[0028] Example 1: Synthesis of 2-[2-(4-dimethylamino-phenyl)-vinyl]-1,3-dimethyl-3H-benzimidazole iodide (DI)
[0029] Dissolve 1.32g (10mmol) of 2-methylbenzimidazole and 2.83g (20mmol) of methyl iodide in 50ml of toluene, stir for 6 hours, then reflux for 30 minutes, cool to room temperature, filter, and wash with ether to obtain N-formaldehyde Base-2-methylbenzimidazole iodide salt. Dissolve 0.288g (1mmol) of N-methyl-2-methylbenzimidazole iodide salt and 0.149g (1mmol) of p-dimethylaminobenzaldehyde in 20ml of methanol, drop 3 drops of piperidine, and reflux for 6h without Washed with water and ethanol to obtain a brown powder with a yield of 77%.
[0030] 1 H NMR (400MHz, DMSO-d 6 ), δ (ppm): 7.987 (d, J = 3.16Hz, 2H), 7.796 (t, J = 6.6Hz, 2H), 7.721 (s, 1H), 7.650 (dd, J 1 =J 2 =3.08Hz,2H),7.213(d,J=16.48,1H),6.822(d,J=8.84,2H),4.107(s,6H),3.044(s,6H). 13 C NMR (400MHz, DMSO-d 6 ), δ (ppm): 152.79, 149.60, 147.58, 132.46, 131.06, 126.40, 122.19, 112.99, 112.15, 10...
Embodiment 2
[0031] Example 2: Synthesis of 2-[2-(4-dimethylamino-phenyl)-vinyl]-1,3-dibutyl-3H-benzimidazole iodide salt
[0032]1.32g (10mmol) 2-methylbenzimidazole and 3.68g (20mmol) iodobutane were dissolved in 50ml of toluene, stirred for 6 hours, then refluxed for 30 minutes, cooled to room temperature, filtered, and washed with ether to obtain N- Butyl-2-methylbenzimidazole iodide salt. Dissolve 0.372g (1mmol) of N-butyl-2-methylbenzimidazole iodide salt and 0.149g (1mmol) of p-dimethylaminobenzaldehyde in 20ml of methanol, add 3 drops of piperidine dropwise, and reflux for 6h. Washed with water and ethanol to obtain a brown powder with a yield of 70%.
Embodiment 3
[0033] Example 3: Synthesis of 2-[2-(4-dimethylamino-phenyl)-vinyl]-1,3-diethylethyl ether-3H-benzimidazolium bromide
[0034] Dissolve 1.32g (10mmol) 2-methylbenzimidazole and 3.06g (20mmol) 2-bromoethyl ethyl ether in 50ml of toluene, stir for 6 hours, then reflux for 30 minutes, cool to room temperature, filter, diethyl ether After washing, N-ethyl ethyl ether-2-methylbenzimidazolium bromide was obtained. Dissolve 0.404g (1mmol) N-ethyl ethyl ether-2-methylbenzimidazolium bromide and 0.149g (1mmol) p-dimethylaminobenzaldehyde in 20ml methanol, and add 3 drops of piperidine dropwise, and reflux After reacting for 7 hours and washing with absolute ethanol, a brown powder was obtained with a yield of 65%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com