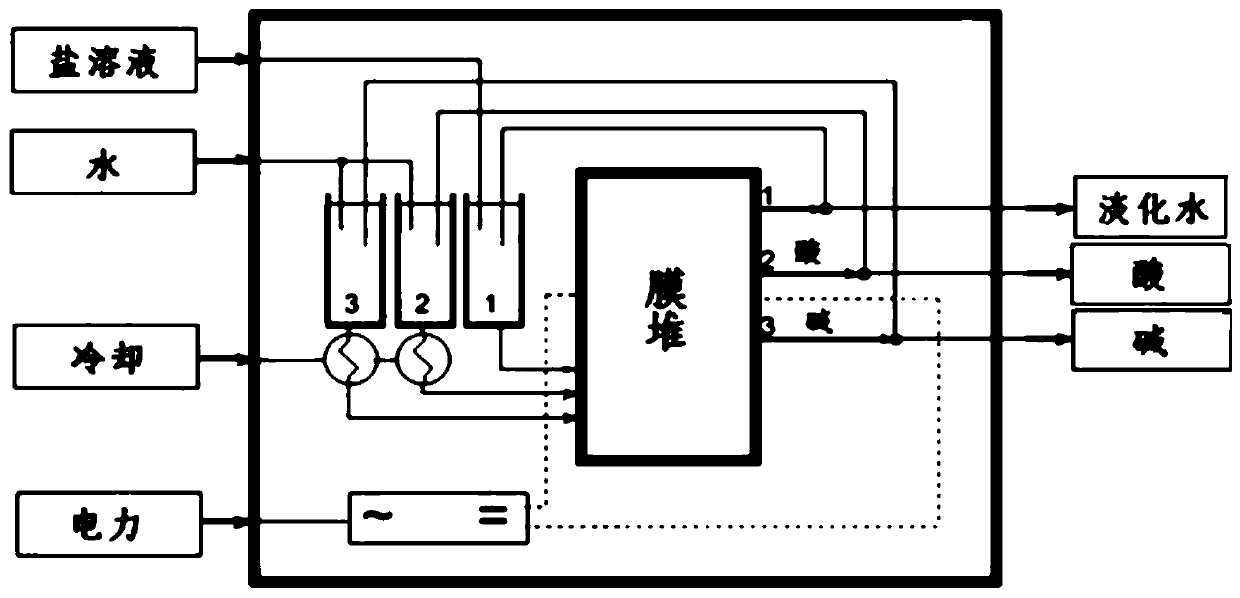
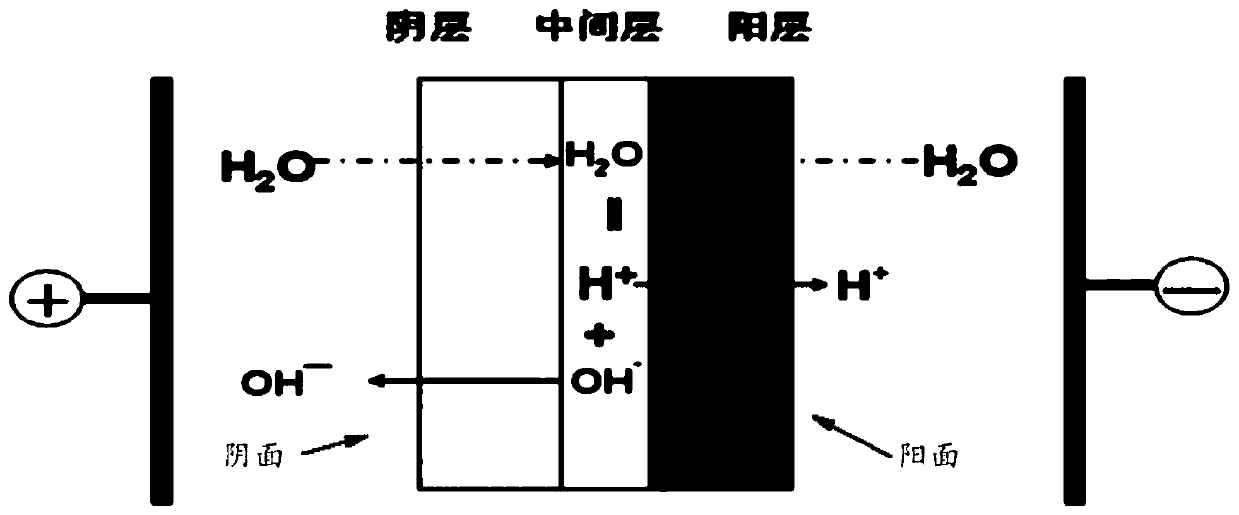
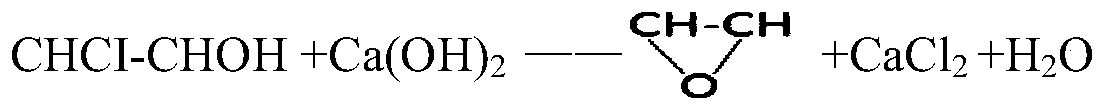A method for preparing haloethanol and ethylene oxide from dry gas
A technology of ethylene oxide and haloethanol, which is applied in the preparation of introducing hydroxyl and halogen, organic chemistry, production of bulk chemicals, etc., can solve the problems of dangerous production, environmental pollution, easy to cause explosion, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0097] A method for preparing haloethanol from dry gas, the method may further comprise the steps:
[0098] (1) Haloalcoholization: Add 70wt% concentration of hydrogen peroxide H 2 o 2 Aqueous solution, 35wt% concentration of HCl solution (hydrochloric acid) and dry gas (the volume content of ethylene is 33.9vol%), at a temperature of 45 ℃, carry out chlorohydrinization reaction, wherein 70wt% concentration of hydrogen peroxide H 2 o 2 , 35wt% concentration of HCl solution and the flow rate of dry gas should make H 2 o 2 The volume ratio of HCl to dry gas is about 1.1:1.5:1. Chlorohydrin is obtained.
Embodiment 2
[0103] A method for preparing haloethanol from dry gas, the method may further comprise the steps:
[0104] 1) Haloalcoholization: add tungstic acid catalyst, 35wt% concentration of hydrogen peroxide H in the tower reactor 2 o 2 , HCl solution (hydrochloric acid) and dry gas of 20wt% concentration, carry out chlorohydrinization reaction at the temperature of 35 ℃, wherein the mass ratio of tungstic acid catalyst and dry gas is 0.05:1, the hydrogen peroxide H of 35wt% concentration 2 o 2 , 20wt% concentration of HCl solution and the addition of dry gas should make H 2 o 2 The volume ratio of HCl to dry gas is about 2:3:1. Chlorohydrin is obtained.
Embodiment 3
[0112] A method for preparing ethylene oxide with dry gas, the method comprises the following steps:
[0113] (1) Halohydrin: Add tungstic acid catalyst and hydrogen peroxide H with a concentration of 35 wt% to the tower reactor 2 O 2 , 20wt% concentration of HCl solution (hydrochloric acid) and dry gas, chlorohydrin reaction is carried out at a temperature of 35 ℃, wherein 35wt% concentration of hydrogen peroxide H 2 O 2 , 20wt% concentration of HCl solution and the flow rate of dry gas alkene should be such that H 2 O 2 The volume ratio of HCl to dry gas is approximately 1.1:1.5:1. Obtain chloroethanol.
[0114] (2) Saponification: The chloroethanol obtained in step (1) is subjected to a saponification reaction with sodium hydroxide to obtain an organic phase of ethylene oxide and a sodium chloride solution. The saponification reaction was carried out in a steel tower reactor, and the upper part was designed as a sieve tray tower. The steam enters from the bottom of t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com