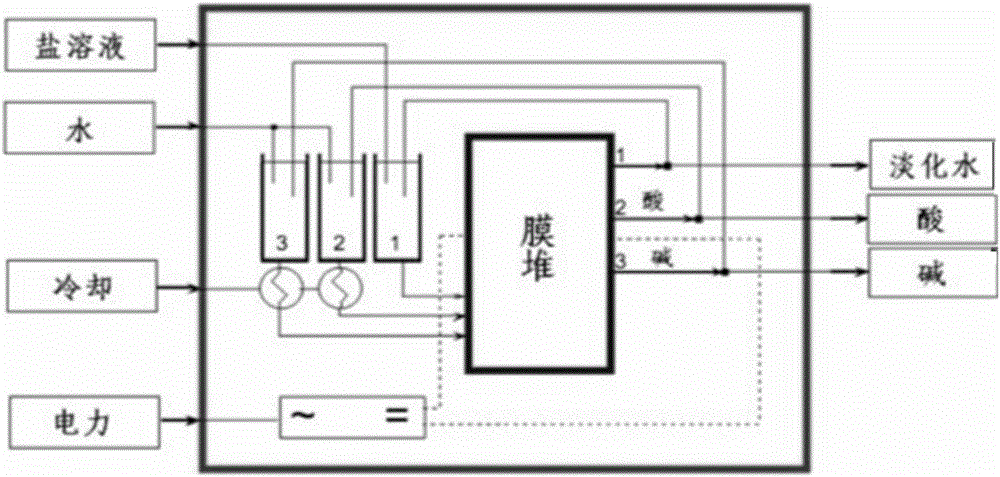
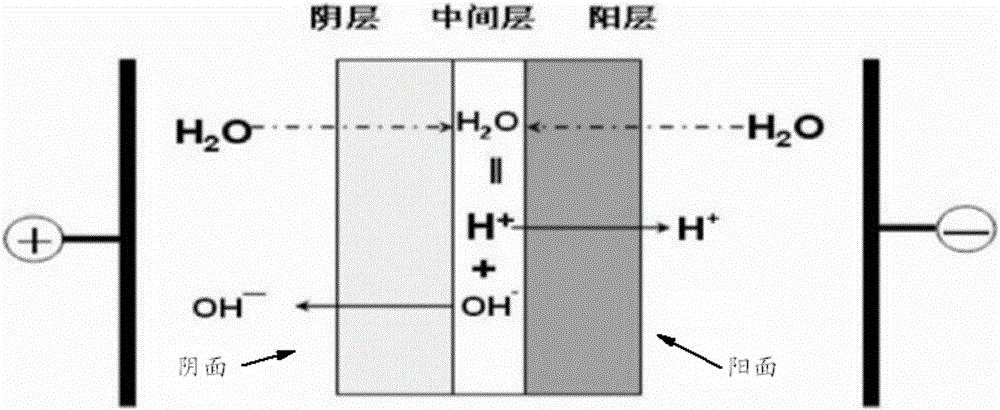
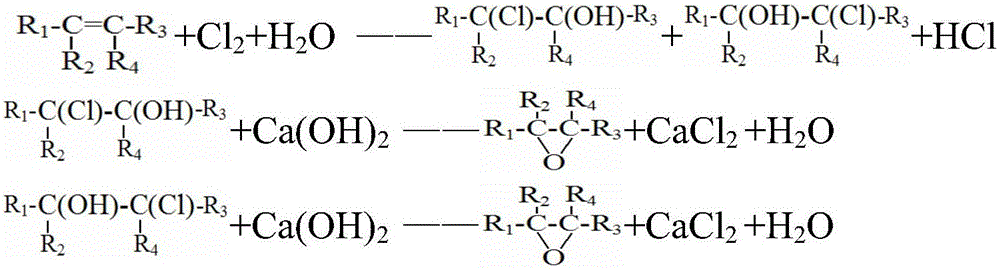
Method for preparing halogenohydrin and epoxide
An epoxide and hydroxide technology, applied in chemical instruments and methods, separation methods, introduction of hydroxyl and halogen preparations, etc., can solve the problems of dangerous production, environmental pollution, easy to cause explosions, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment A
[0100] 1) Haloalcoholization: add water, chlorine (Cl 2 ) and propylene, carry out chloroalcoholization reaction at a temperature of 45°C, and the residence time in the tubular reactor is 30 minutes, wherein chlorine (Cl 2 ) and propylene flow should be such that liquid chlorine (Cl 2 ) to propylene in a molar ratio of 3.4:1, and the amount of water introduced is 99 times the mass of propylene. This gives the halohydrin, a mixture of 2-chloropropan-1-ol and 1-chloropropan-2-ol. Wherein the halohydrin contains 9wt% halogenated hydrocarbon by-products.
[0101] 2) Saponification: performing saponification reaction with the haloalcohol obtained in step 1) and sodium hydroxide, and separating to obtain the propylene oxide organic phase and sodium chloride solution. The saponification reaction is carried out in a steel tower reactor, and the upper part is designed as a sieve tray tower. Steam enters from the bottom of the tower to blow out the crude propylene oxide generated fr...
Embodiment 1
[0108] 1) Haloalcoholization: Add 70wt% concentration of hydrogen peroxide H 2 o 2 , HCl solution (hydrochloric acid) and propylene of 35wt% concentration, carry out chlorohydrinization reaction at the temperature of 45 ℃, wherein the hydrogen peroxide H of 70wt% concentration 2 o 2 , 35wt% concentration of HCl solution and propylene three flow should make H 2 o 2 The molar ratio of HCl to propylene is about 1.2:1.2:1. This gives the halohydrin, a mixture of 2-chloropropan-1-ol and 1-chloropropan-2-ol.
Embodiment 2
[0112] 1) Haloalcoholization: add tungstic acid catalyst, 35wt% concentration of hydrogen peroxide H in the tower reactor 2 o 2 , HCl solution (hydrochloric acid) and propylene of 20wt% concentration, carry out chlorohydrinization reaction at the temperature of 35 ℃, wherein the mass ratio of tungstic acid catalyst and propylene is 0.05:1, the hydrogen peroxide H of 35wt% concentration 2 o 2 , the addition amount of HCl solution of 20wt% concentration and propylene should make H 2 o 2 The molar ratio of HCl to propylene is about 1.5:1.1:1. This gives the halohydrin, a mixture of 2-chloropropan-1-ol and 1-chloropropan-2-ol.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com