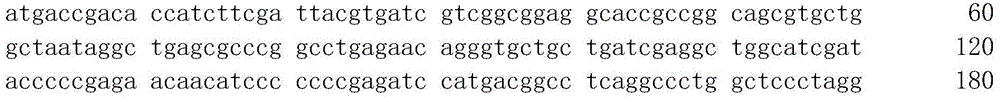Glycosylated 5-hydroxymethyl furfural oxidase and preparation thereof
A hydroxymethylfurfural oxidase, glycosylation technology, applied in the directions of oxidoreductase, microorganism-based methods, biochemical equipment and methods, etc., can solve the problems of low expression, no active inclusion bodies, etc., to avoid toxins The effect of producing, improving purity, and increasing yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] 1. With the 5-hydroxymethylfurfural oxidase gene (see the sequence table SEQ ID NO: 1 for the sequence), after NadeI and XhoI double-digestion (the gene is on the pUC18 plasmid, the plasmid is double-digested, plasmid 36ul, NdeI and 3ul each of XhoI, plus 5ul of 10x H buffer. Enzyme digestion reaction conditions, 37°C, 3h). Then, the cleaved gene fragment was recovered by 1% agarose electrophoresis (using a fragment recovery kit, Axygen Company, Cat. No. AP-GX-50). The purified fragment was ligated to the PET28a carrier with double enzyme digestion under the same conditions, and ligated overnight at 16°C with T4 ligase (the composition of each material in the reaction system: 8ul plasmid, 1ul reaction buffer, 1ul T4 ligase). Transform Escherichia coli Top10 competent that was frozen in our laboratory. Take 5ul of the connection solution and add it to 100ul competent cells, mix on ice for 10 minutes, place in a 42°C water bath for 45 seconds, and place on ice for 2 minu...
Embodiment 2
[0039] The optimization of gene codon mainly considers the following factors:
[0040] 1. The expression of foreign genes in Pichia pastoris is also closely related to the selection of gene codons. The codon usage bias of Pichia pastoris has important implications for the regulation of HMFO gene expression from translation. Pichia pastoris codon preference, by analyzing the synonymous codon usage of 30 protein-coding genes of Pichia pastoris and calculating the codon usage of the yeast, the high-expression superior codons of Pichia pastoris were determined.
[0041] 2. To balance GC content, genes with high AT content often cannot be efficiently transcribed due to premature termination. Early termination is a species-specific phenomenon. GC content should be controlled between 40-60%.
[0042]3. mRNA secondary structure, stable mRNA secondary structure and molecules near the 5' end also have an important impact on gene expression. Using the open reading frame upstream of t...
Embodiment 3
[0047] 1. The 5-hydroxymethylfurfural oxidase gene is codon-optimized (see the sequence listing SEQ ID NO: 1 for the sequence), after double digestion with restriction endonucleases XhoI and XbaI (the gene is on the pUC18 plasmid, and the plasmid enzyme Cut into components, plasmid 36ul, XbaI and XhoI 3ul each, add 10x H buffer 5ul. Enzyme digestion reaction conditions, 37°C, 3h). Then, the cleaved gene fragment was recovered by 1% agarose electrophoresis (using a fragment recovery kit, Axygen Company, Cat. No. AP-GX-50). The purified fragment was ligated to the pPICZa-A vector with double enzyme digestion under the same conditions, and ligated overnight at 16°C by T4 ligase (the composition of each material in the reaction system: 8ul plasmid, 1ul reaction buffer, 1ul T4 ligase). Transform Escherichia coli Top10 competent that was frozen in our laboratory. Take 5 ul of the connection solution and add it to 100 ul of competent cells Escherichia coli TOP10, mix on ice for 10 m...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap