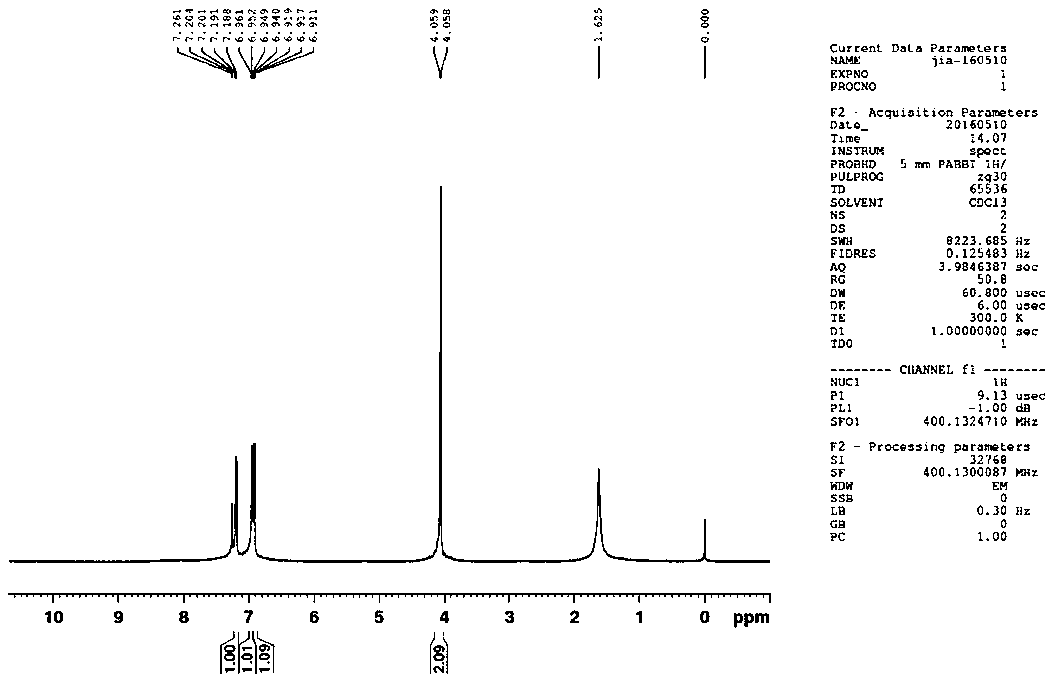
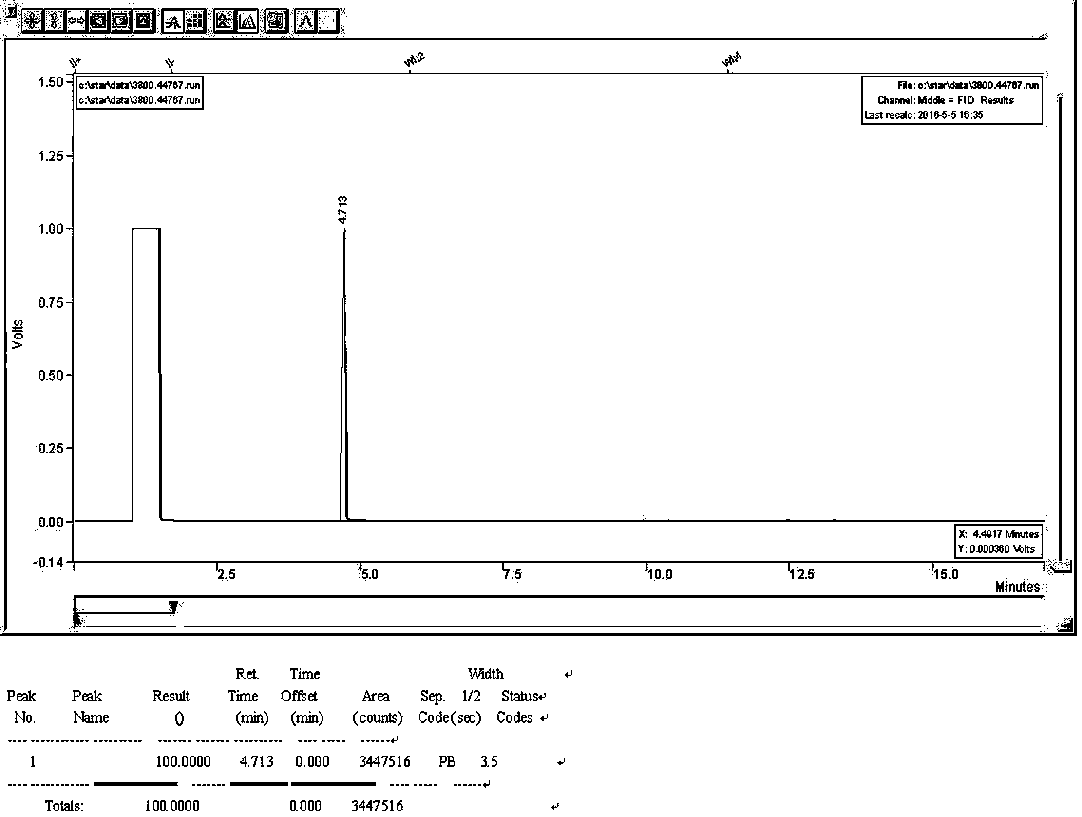
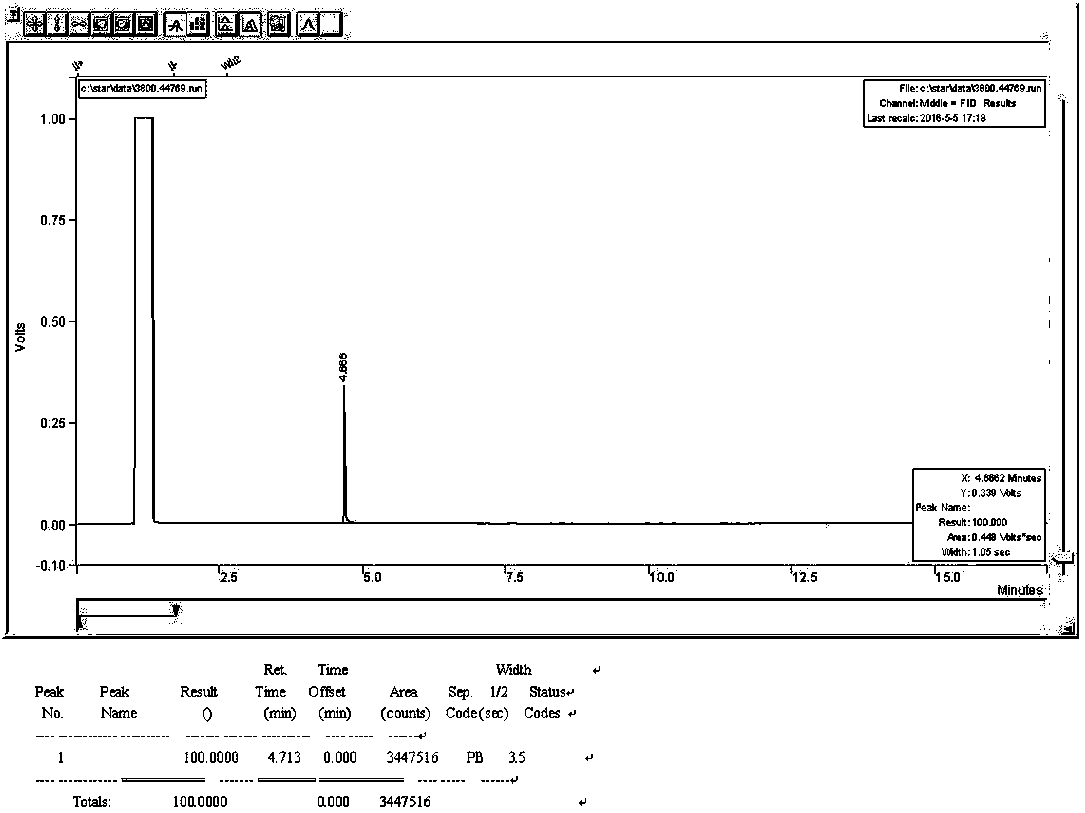
A kind of synthetic method of 2-thiophene methylamine
A technology of thiophene methylamine and a synthesis method, which is applied in the direction of organic chemistry and the like, can solve the problems of unobtainable raw materials, expensive reducing agents and catalysts, etc., and achieves the effects of cheap raw materials, excellent quality, and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Embodiment one: drop into 44.0 grams (0.392 moles) 2-thiophene formaldehyde, 240 milliliters of ethanol, 32.7 grams (0.47 moles, Excess 20%) hydroxylamine hydrochloride and 30 ml of water, stirred for 60 minutes to completely dissolve.
[0051]Under the water bath, add 196 grams (1.96 moles, 5 times the amount) of 36.5% hydrochloric acid dropwise, while slowly adding 77.2 grams (1.18 moles, 3 times the amount) of zinc powder, and control the reaction temperature at 20-30 ° C. After feeding, continue stirring After 100 minutes, add 151 ml (about 2.12 moles, 1.8 times the amount of zinc powder) of 25-28% ammonia solution, and continue to add 70.56 grams (1.764 moles, 0.9 times the amount of acid) of sodium hydroxide and 282 The solution prepared in milliliters of water was continued to stir for 60 minutes, then suction filtered, the filter cake was washed with about 300 milliliters of ethanol and then drained, the filtrate and the ethanol washing liquid were combined and ...
Embodiment 2
[0053] Embodiment two: drop into 44.0 grams (0.392 moles) 2-thiophene formaldehyde, 400 milliliters of ethanol, 31.3 grams (0.451 moles, Excess 15%) hydroxylamine hydrochloride and 30 ml of water, stirred for 60 minutes to completely dissolve. Under the water bath, add 176.4 grams (about 1.764 moles, 4.5 times the amount) of 36.5% hydrochloric acid dropwise, while slowly adding 70.6 grams (1.08 moles, 2.75 times the amount) of zinc powder, and control the reaction temperature at 30-40 ° C. After feeding, continue Stir for 60 minutes, add 153.8 ml (about 2.16 moles, 2.0 times the amount of zinc powder) of 25-28% ammonia solution, and continue to add 70.56 grams (1.764 moles, 1.0 times the amount of acid) of sodium hydroxide and The solution prepared with 141 ml of water, continue to stir for 60 minutes, then filter with suction, wash the filter cake with about 200 ml of ethanol and drain it, combine the filtrate and ethanol washing solution, extract with 80 ml*4 dichloromethane...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com