Recombinant expression vector, engineering bacteria, preparation method and application thereof
A recombinant vector and engineering bacteria technology, applied in the field of genetic engineering, can solve the problems of low purity, complex IL-18 purification process, low recovery, etc., and achieve the effects of high purity, high recovery rate and simple process characteristics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
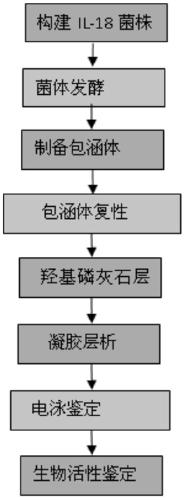
[0090] The invention provides a recombinant expression vector, engineering bacteria, preparation method and application thereof. The present invention utilizes gene recombination technology to construct a genetically engineered bacterium that highly expresses human IL-18, establishes a simple and effective technology for purifying IL-18, solves the problem of preparing a large amount of IL-18, and can meet future clinical needs. A stable and simple process route for inclusion body renaturation and IL-18 purification has been established, and the protein purity can reach more than 97%; a method for identifying IL-18 activity has been established.
[0091] The invention mainly solves the problems of complicated IL-18 purification process, low recovery and low purity. The process features simplicity, rapidity, high recovery rate and high purity (97%).
[0092] The invention also provides a method for detecting IL-18 activity. The identification of interleukin-18 activity usually ...
Embodiment 1
[0095] Example 1 Construction of the prokaryotic expression vector pBV220-IL-18 of IL-18
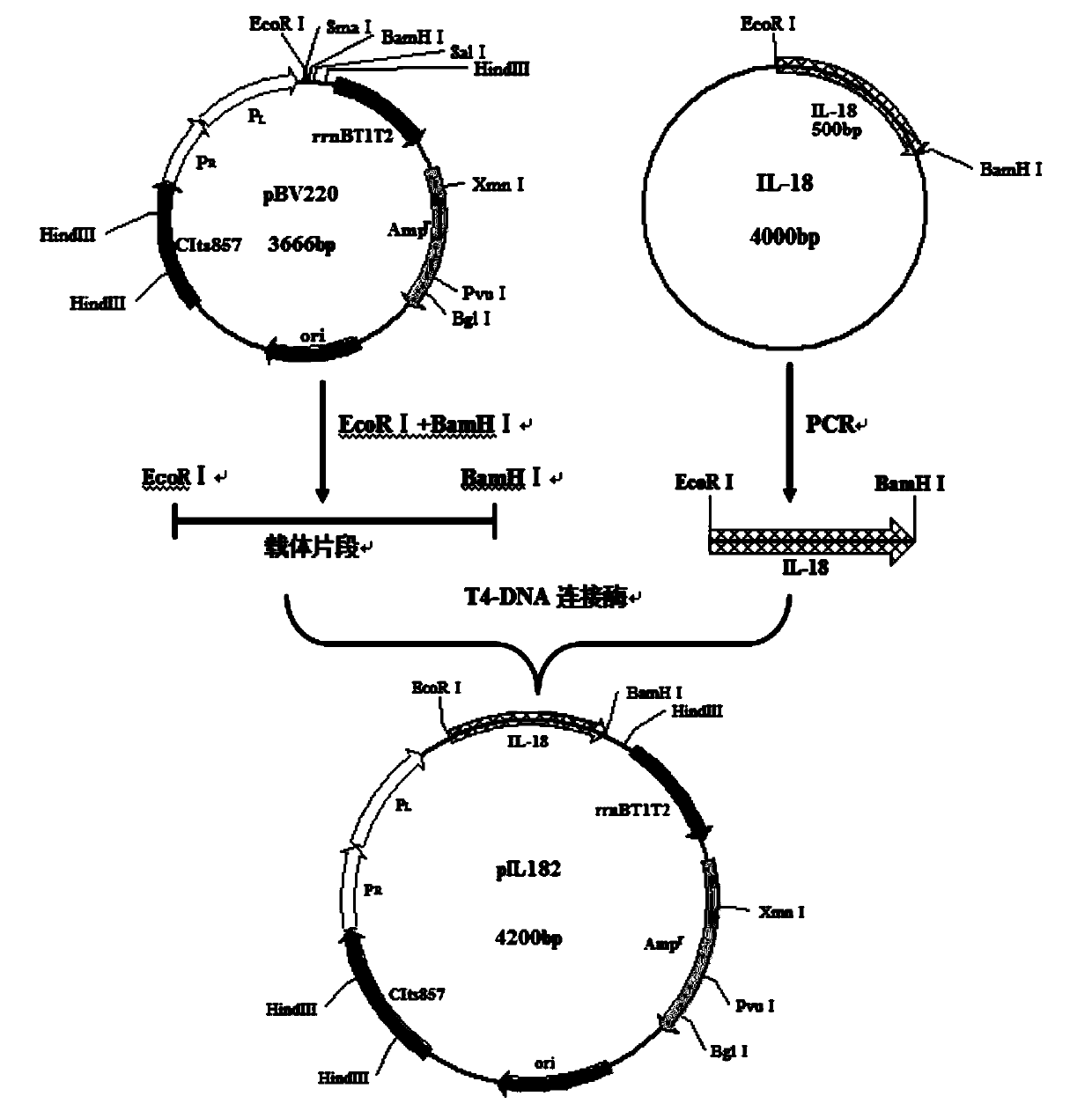
[0096] The gene containing IL-18 obtained by RT-PCR was digested with EcoRI and BamHI, and then ligated with the vector pBV220 which was digested with EcoRI and BamHI; the prokaryotic expression vector pBV220-IL-18 containing the IL-18 gene was constructed, Transform Escherichia coli DH5α to construct engineering strain DH5α-pBV220 IL-18; (see figure 2 , 3 ).
Embodiment 2
[0097] Fermentation and high-efficiency expression of embodiment 2hrIL-18
[0098] Activation, fermentation, induction and recovery of inclusion bodies of engineered bacteria: inoculate engineered strains stored at -70°C on nutrient agar plate medium containing Amp, and culture at 37°C for more than 12 hours; pick a single colony and inoculate into LB containing Amp In the liquid medium, culture on a shaking table at 37°C for more than 12 hours; inoculate the bacterial liquid into the fermentation medium containing Amp at a ratio of 1-10%, and cultivate it on a shaking table at 28-32°C for more than 12 hours; inoculate the bacterial liquid at a ratio of 5-10% Proportionally inoculated into the fermenter and cultured at 28-32°C; wait until the concentration of the bacterial solution reaches OD 600 After about 2.0-3.0, the temperature was raised to 42°C to induce culture for 3-6 hours, and at the same time, a small amount of filler solution was continuously added, and the ammoni...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com