Application of o-hydroxyphenylazole derivatives as organic blue light materials
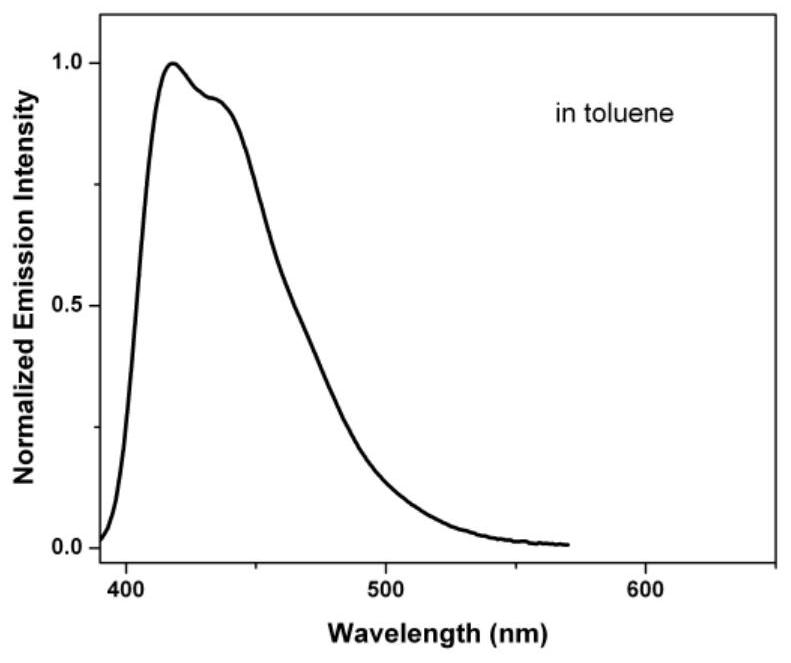
A technology of organic molecular materials and blue light, applied in luminescent materials, organic chemistry, chemical instruments and methods, etc., can solve the problems of luminescent color purity, quantum efficiency and low stability, and achieve high fluorescence quantum efficiency and good thermal stability , good effect of color purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
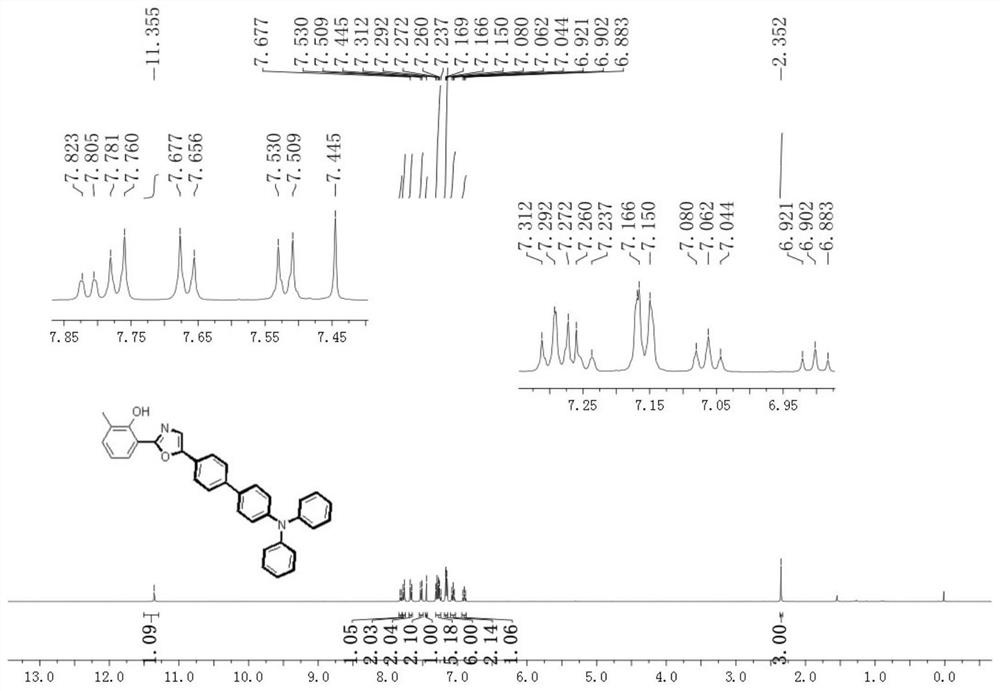
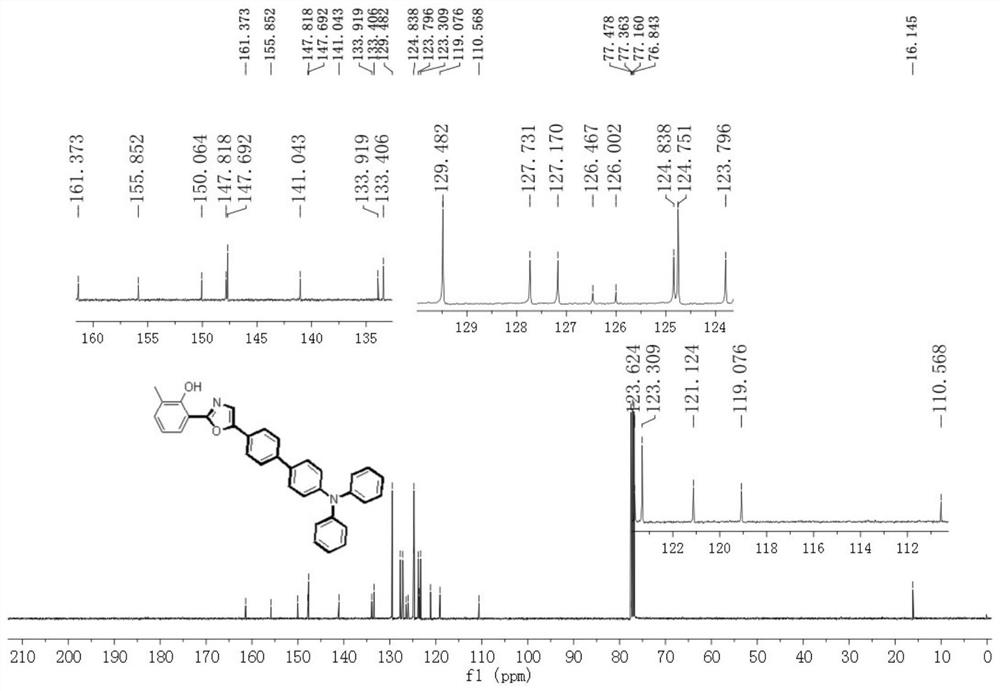
[0029] Example 1: Synthesis of 2-(5-(4'-(diphenylamino)-[1,1'-diphenyl]-4-)oxazol-2-yl)-6-methylphenol
[0030]Under nitrogen protection, o-methylphenoxyacetamide (0.2mmol, 33.2mg), 4'-(5-oxazolyl)-N,N-diphenyl-[1,1'-diphenyl] -4-amine (0.3mmol, 116.4mg), dichloro(pentamethylcyclopentadienyl) rhodium (III) dimer (5.0mol%, 6.4mg), silver hexafluoroantimonate (20mol%, 13.6mg), pivalic acid (0.4mmol, 41.0mg), cesium pivalate (0.16mmol, 38mg), silver carbonate (0.08mmol, 22mg) were added to a reaction tube equipped with a magnetic stirrer, and added under nitrogen N,N-Dimethylformamide (1.0 mL) was stirred at room temperature for 5 minutes, and then reacted at 140° C. for 24 hours. After the reaction was completed, the reaction tube was cooled to room temperature, ethyl acetate was added to dilute the reaction system, filtered through diatomaceous earth, and washed with ethyl acetate, the filtrates were combined, the solvent was removed under reduced pressure, and the residue was...
Embodiment 2
[0031] Example 2: Synthesis of 2-(5-(4'-(diphenylamino)-[1,1'-diphenyl]-4-)oxazol-2-yl)-4-methylphenol
[0032] Under nitrogen protection, p-methylphenoxyacetamide (0.2mmol, 33.2mg), 4'-(5-oxazolyl)-N,N-diphenyl-[1,1'-diphenyl] -4-amine (0.3mmol, 116.4mg), dichloro(pentamethylcyclopentadienyl) rhodium (III) dimer (5.0mol%, 6.4mg), silver hexafluoroantimonate (20mol%, 13.6mg), pivalic acid (0.4mmol, 41.0mg), cesium pivalate (0.16mmol, 38mg), silver carbonate (0.08mmol, 22mg) were added to a reaction tube equipped with a magnetic stirrer, and added under nitrogen N,N-Dimethylformamide (1.0 mL) was stirred at room temperature for 5 minutes, and then reacted at 140° C. for 24 hours. After the reaction was completed, the reaction tube was cooled to room temperature, ethyl acetate was added to dilute the reaction system, filtered through diatomaceous earth, and washed with ethyl acetate, the filtrates were combined, the solvent was removed under reduced pressure, and the residue wa...
Embodiment 3
[0033] Example 3: 2-(5-(4'-(diphenylamino)-[1,1'-diphenyl]-4-)oxazol-2-yl)-6-trifluoromethylphenol Synthesis
[0034] Under nitrogen protection, p-trifluoromethylphenoxyacetamide (0.2mmol, 43.8mg), 4'-(5-oxazolyl)-N,N-diphenyl-[1,1'-diphenyl Base]-4-amine (0.3mmol, 116.4mg), dichloro(pentamethylcyclopentadienyl) rhodium (III) dimer (5.0mol%, 6.4mg), silver hexafluoroantimonate (20mol %, 13.6mg), pivalic acid (0.4mmol, 41.0mg), cesium pivalate (0.16mmol, 38mg), silver carbonate (0.08mmol, 22mg) were added to the reaction tube equipped with a magnetic stirrer, under nitrogen N,N-dimethylformamide (1.0 mL) was added under low temperature, stirred at room temperature for 5 minutes, and then reacted at 140° C. for 24 hours. After the reaction was completed, the reaction tube was cooled to room temperature, ethyl acetate was added to dilute the reaction system, filtered through diatomaceous earth, and washed with ethyl acetate, the filtrates were combined, the solvent was removed ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thermal decomposition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com