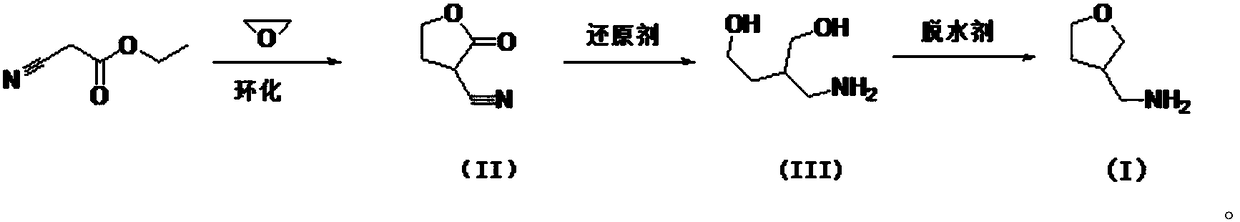
A kind of preparation method of 3-aminomethyltetrahydrofuran
A technology of aminomethyl and cyano groups, applied in the field of compound synthesis, can solve the problems of difficulty in synthesizing tetrahydrofuran compounds, unfavorable industrial production, long steps, etc., and achieve the effects of low production cost, low environmental pollution, and high utilization rate of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Embodiment 1: (1) cyclization reaction
[0023]
[0024] Weigh 7.14g (0.105mol) of sodium ethoxide solid into a 250ml four-necked bottle, add 50ml of absolute ethanol to dissolve, add 11.3g (0.1mol) of ethyl cyanoacetate at 25°C, and stir at 25°C for 1 hour , a large amount of white solid appeared.
[0025] At room temperature, 5.54 g (0.126 mol) of ethylene oxide was introduced into the four-neck flask. After the introduction, the temperature was raised to 45-50° C. and stirred for 4 hours. The white solid disappeared and the system became an amber clear solution.
[0026] The whole system is subjected to vacuum distillation to remove absolute ethanol; for the distillation residue, add 50ml of pure water to the distillation residue, and adjust the pH value to 2-3 with concentrated hydrochloric acid; and extract with ethyl acetate (50ml*2) , combined the organic phases, evaporated under reduced pressure to remove ethyl acetate, and the residue continued to be rectif...
Embodiment 2
[0036] (1) Cyclization reaction
[0037] Weigh 11.78g (0.105mol) of potassium tert-butoxide solid into a 250ml four-necked bottle, add 50ml of tert-butanol to dissolve, add 11.3g (0.1mol) of ethyl cyanoacetate at 20°C, and stir at 20°C After 1 hour, a large amount of white solid appeared.
[0038] At room temperature, 5.54 g (0.126 mol) of ethylene oxide was introduced into the four-necked flask. After the introduction, the temperature was raised to 45-50° C. and stirred for 3 hours. The white solid disappeared, and the system became an amber clear solution.
[0039] The whole system is subjected to vacuum distillation to remove tert-butanol; for the distillation residue, add 50ml of pure water to the distillation residue, and adjust the pH value to 2-3 with concentrated hydrochloric acid; and extract with ethyl acetate (50ml*2) , combined the organic phases, evaporated under reduced pressure to remove ethyl acetate, and the residue continued to be rectified under reduced pre...
Embodiment 3
[0046] (1) Cyclization reaction
[0047] Weigh 7.14g (0.105mol) of sodium ethoxide solid into a 250ml four-necked bottle, add 50ml of absolute ethanol to dissolve, add 11.3g (0.1mol) of ethyl cyanoacetate at 25°C, and stir at 25°C for 1 hour , a large amount of white solid appeared.
[0048] Pass 5.54 g (0.126 mol) of ethylene oxide into the four-necked flask at 25°C. After the pass-in is complete, raise the temperature to 45-50°C and stir for 3 hours. The white solid disappears, and the system turns into an amber clear solution.
[0049] The whole system is subjected to vacuum distillation to remove absolute ethanol; for the distillation residue, add 50ml of pure water to the distillation residue, and adjust the pH value to 2-3 with concentrated hydrochloric acid; and extract with ethyl acetate (50ml*2) , combined the organic phases, evaporated under reduced pressure to remove ethyl acetate, and the residue continued to be rectified under reduced pressure; combined the organ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com