1,1-dipyrazolemethane binuclear manganese polymer and its in-situ decarboxylation synthesis method and application
A dipyrazolemethane and synthesis method technology, which is applied in the field of in-situ decarboxylation synthesis of 1,1-dipyrazolemethane binuclear manganese polymers, can solve the problems of low conversion rate and slow reaction speed, and achieve low cost and good conditions Gentle and stable effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
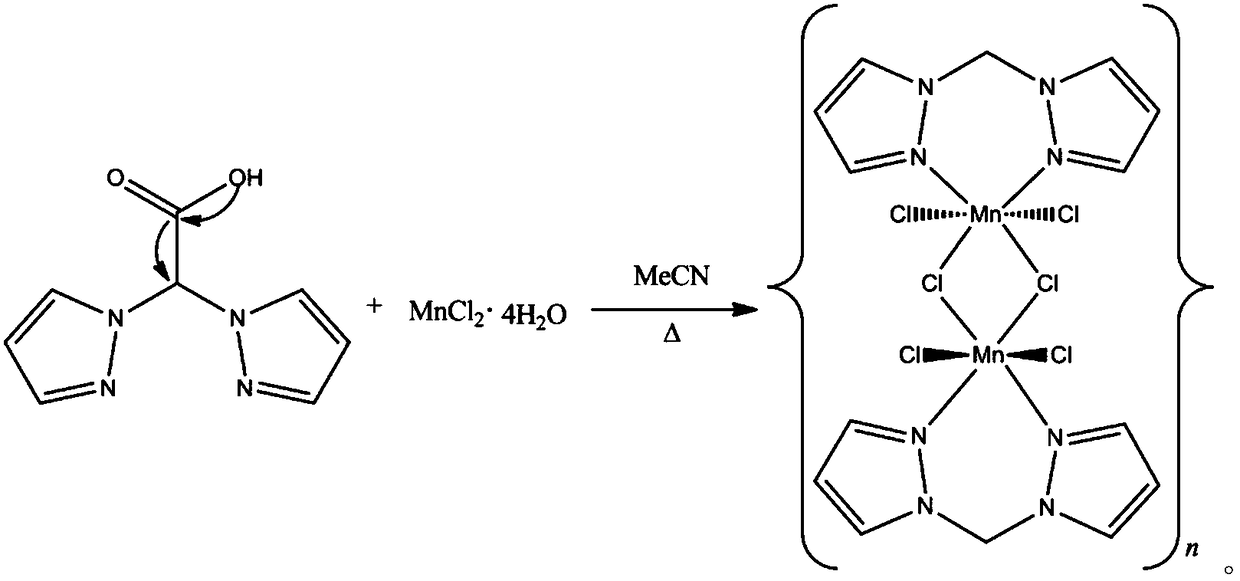
[0025] (1) 0.197 grams of analytically pure MnCl 2 4H 2 O and 0.192 grams of analytically pure 1,1-dipyrazole acetic acid were dissolved in 10 milliliters of anhydrous acetonitrile;
[0026] (2) The solution in step (1) was stirred at room temperature for 5 minutes until all reactants were dissolved and clarified;
[0027] (3) Transfer the solution prepared in step (2) to a polytetrafluoroethylene reactor and react at 110°C for 100 hours, cool down to room temperature, open the kettle, filter, and wash with anhydrous acetonitrile to obtain single crystal grade 1,1 -Dipyrazolemethane binuclear manganese polymer, yield 78-85%.
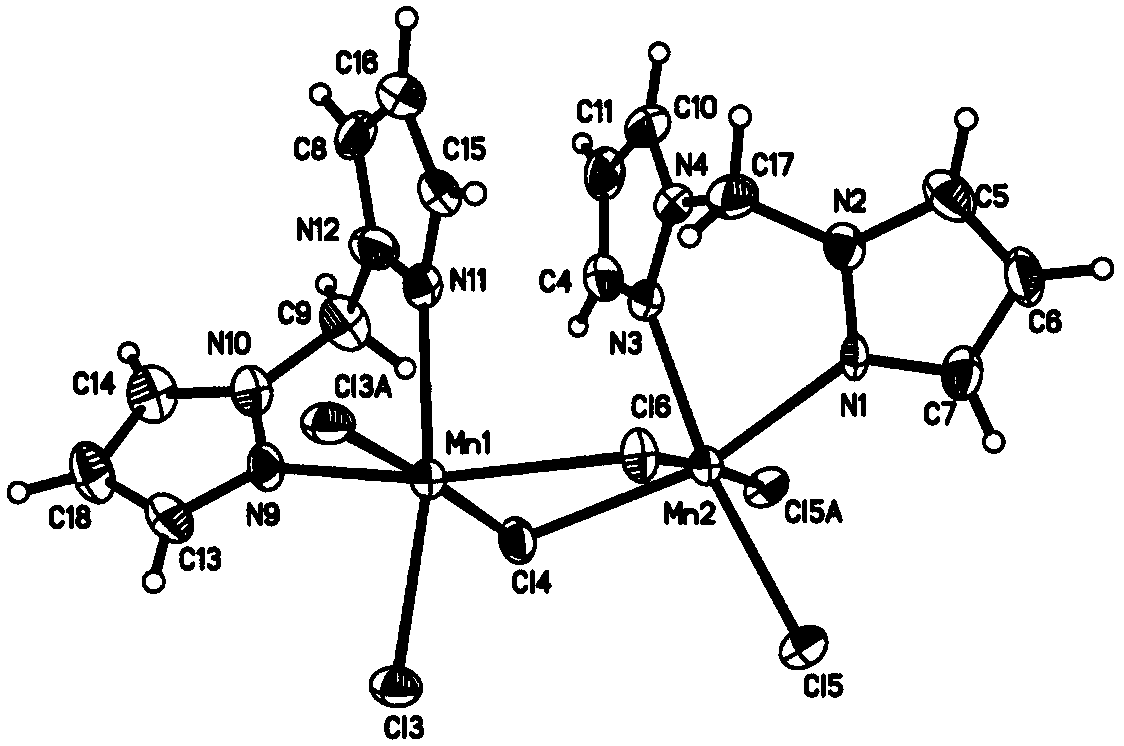
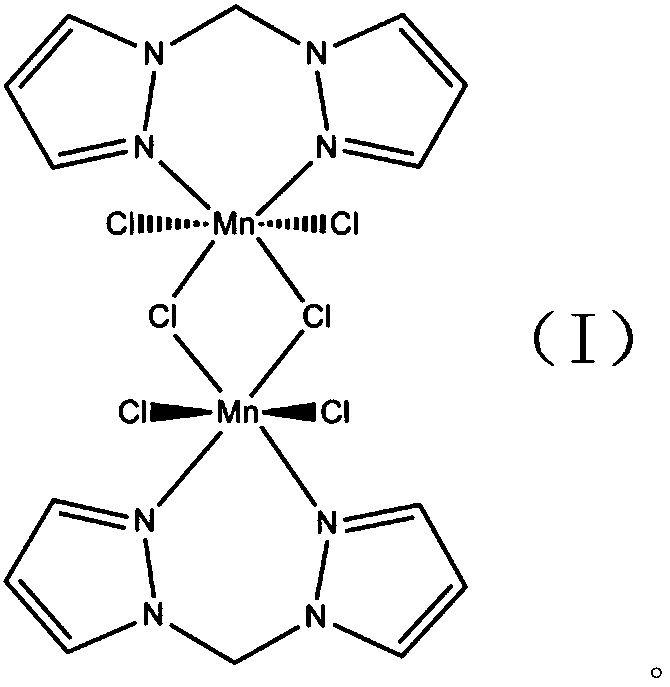
[0028] And confirm its structural formula with X-ray single crystal diffraction, infrared spectrum, mass spectrum, nuclear magnetic resonance proton spectrum / carbon spectrum as:
[0029]
[0030] The basic data of the resulting 1,1-dipyrazolemethane binuclear manganese polymers were analyzed:
[0031] X-ray single crystal diffraction characterizat...
Embodiment 2
[0033] (1) 0.256 gram of analytically pure MnCl 2 4H 2 O and 0.245 grams of analytically pure 1,1-dipyrazole acetic acid were dissolved in 12 milliliters of anhydrous acetonitrile;
[0034] (2) The solution in step (1) was stirred at room temperature for 5 minutes until all reactants were dissolved and clarified;
[0035] (3) Transfer the solution prepared in step (2) to a polytetrafluoroethylene reactor and react at 120°C for 120 hours, cool down to room temperature, open the kettle, filter, and wash with anhydrous acetonitrile to obtain single crystal grade 1,1 -Dipyrazolemethane binuclear manganese polymer, yield 78-85%.
Embodiment 3
[0037] (1) 0.380 gram of analytically pure MnCl 2 4H 2 O and 0.360 grams of analytically pure 1,1-dipyrazole acetic acid are dissolved in 15 milliliters of anhydrous acetonitrile;
[0038] (2) Stir the solution in step (1) at room temperature for 10 minutes until all reactants are dissolved and clarified;
[0039] (3) Transfer the solution prepared in step (2) to a polytetrafluoroethylene reactor and react at 140°C for 150 hours, cool down to room temperature, open the kettle, filter, and wash with anhydrous acetonitrile to obtain single crystal grade 1,1 -Dipyrazolemethane binuclear manganese polymer, yield 78-85%.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap