Polycyclic aromatic selenide derivatives and preparation method thereof
A technology for polycyclic aromatic selenide and derivatives is applied in the field of polycyclic aromatic selenide derivatives and their preparation, which can solve problems such as group compatibility limitation, achieve high atom economy, complex and diverse structures, and cheap reagents Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
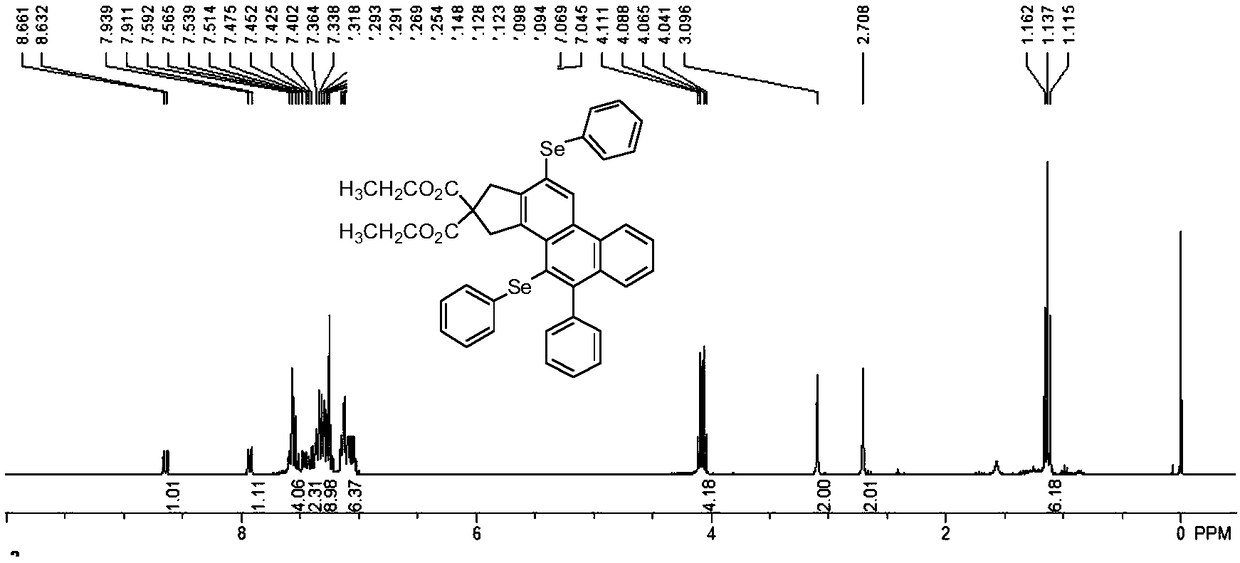
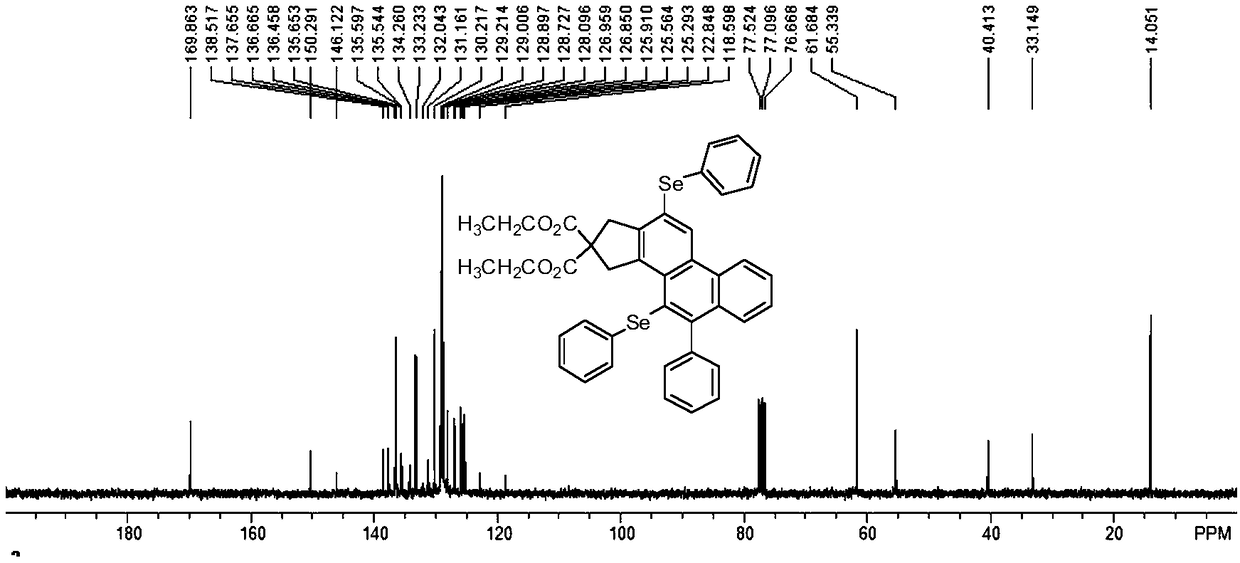
[0053] A polycyclic aromatic selenide derivative provided in this example has a structural formula as shown in formula (I).
[0054]
[0055] This embodiment also provides a method for preparing the above-mentioned polycyclic aromatic selenide derivatives:
[0056] Diethyl malonate bridged diyne compound (20mmol) and phenylacetylene bromide (48mmol) were added to the reaction flask, mixed in Pd(PPh 3 ) 2 Cl 2 and CuI as catalyst in the anhydrous oxygen-free catalytic system, wherein the amount of catalyst is 0.8mmol, Pd(PPh 3 ) 2 Cl 2 :CuI=3:1. With triethylamine (50mmol) as the base and anhydrous acetonitrile (40mL) as the solvent, the reaction was stirred at room temperature for 12 hours, and the reaction product was successively washed with 15% hydrochloric acid solution, 10% sodium bicarbonate solution, and saturated saline, respectively. Washing; then extracted with ethyl acetate, spin-dried under reduced pressure, column chromatography (the volume ratio of ethyl...
Embodiment 2
[0064] This embodiment provides a polycyclic aromatic selenide derivative, the structural formula of which is shown in formula (II).
[0065]
[0066] This embodiment also provides a method for preparing the above-mentioned polycyclic aromatic selenide derivatives:
[0067] Diethyl malonate bridged diyne compound (20mmol) and p-methylphenylacetylene bromide (40mmol) were added to the reaction flask, mixed in Pd(PPh 3 ) 2 Cl 2 and CuI as catalyst in the anhydrous oxygen-free catalytic system, wherein the amount of catalyst is 0.6mmol, Pd(PPh 3 ) 2 Cl 2 :CuI=3.2:1. With triethylamine (60mmol) as the base and anhydrous acetonitrile (25mL) as the solvent, the reaction was stirred at room temperature for 20 hours, and the reaction product was successively washed with 15% hydrochloric acid solution, 10% sodium bicarbonate solution, and saturated saline, respectively. Washing; then extracted with ethyl acetate, spin-dried under reduced pressure, column chromatography (the vo...
Embodiment 3
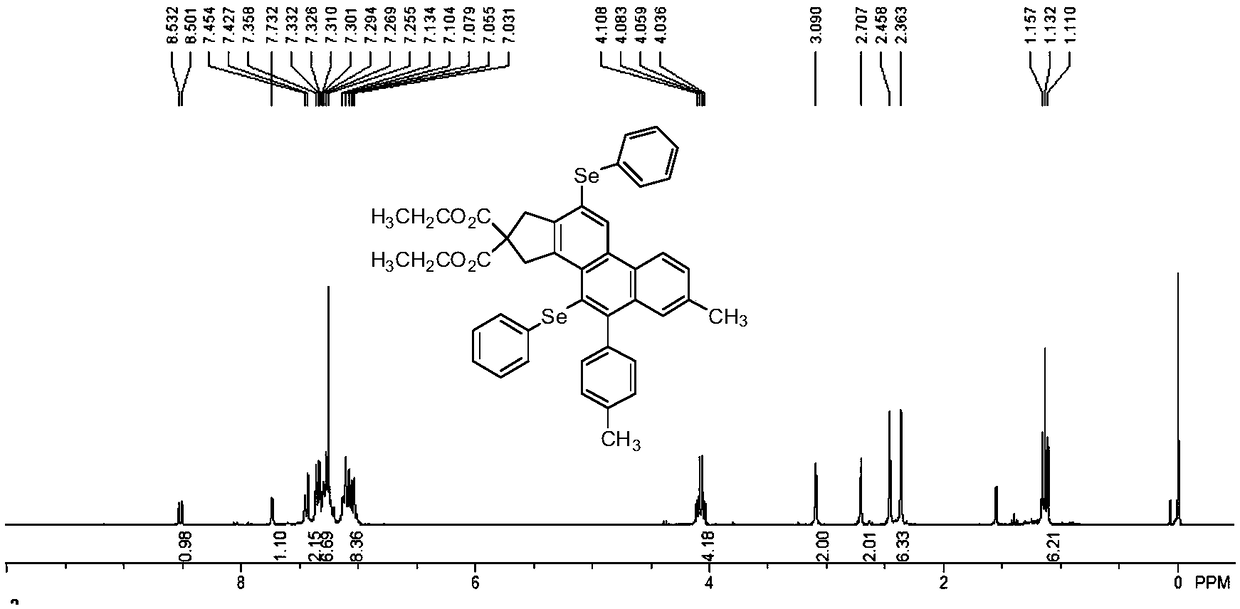
[0075] This embodiment provides a polycyclic aromatic selenide derivative, the structural formula of which is shown in formula (III).
[0076]
[0077] This embodiment also provides a method for preparing the above-mentioned polycyclic aromatic selenide derivatives:
[0078] Diisopropyl malonate bridged diyne compound (20mmol) and p-ethylphenylacetylene bromide (60mmol) were added to the reaction flask, mixed in Pd(PPh 3 ) 2 Cl 2 and CuI as catalyst in the anhydrous oxygen-free catalytic system, wherein the amount of catalyst is 1.0mmol, Pd(PPh 3 )2 Cl 2 :CuI=2.8:1. With triethylamine (100mmol) as the base and anhydrous acetonitrile (33mL) as the solvent, the reaction was stirred at room temperature for 16 hours, and the reaction product was successively washed with 15% hydrochloric acid solution, 10% sodium bicarbonate solution and saturated saline respectively Washing; then extracted with ethyl acetate, spin-dried under reduced pressure, column chromatography (the vo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com