Application of eucheuma polypeptide in prevention and treatment of pulmonary fibrosis
A technology of pulmonary fibrosis and Eucheuma, which is applied in the application field of Eucheuma polypeptide to prevent and treat pulmonary fibrosis, achieving the effect of significantly preventing and controlling pulmonary fibrosis and inhibiting the formation of pulmonary fibrosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] The preparation of embodiment 1 Eucheuma polypeptide
[0041] 1. Preparation of Eucheuma polypeptide
[0042] Take 1 kg of Eucheuma pulveris, homogenate with 10L 20mmol / L HCl solution to prepare a crude liquid, if the solution is thick, add potassium chloride to a concentration of 1.5%; centrifuge the obtained crude liquid at 8000 rpm for 15 minutes, Collect the supernatant, filter the supernatant with three layers of gauze, and separate with dextran G-50 (Sephadex G-50) (the column height is 30 cm, the inner diameter width is 2.0 cm, and the height of the upper sample is 5-10% of the column height) . After equilibrium, elute with 20mmol / L HCl solution at a flow rate of 1.0mL / min, there are two groups of elution peaks, collect the second group of eluted products, adjust the pH of the solution to pH 6.0-7.0, and centrifuge at 10,000 rpm for 15 minutes , and the supernatant was freeze-dried to obtain the desired extract.
[0043] 2. It is confirmed by sodium dodecyl su...
Embodiment 2
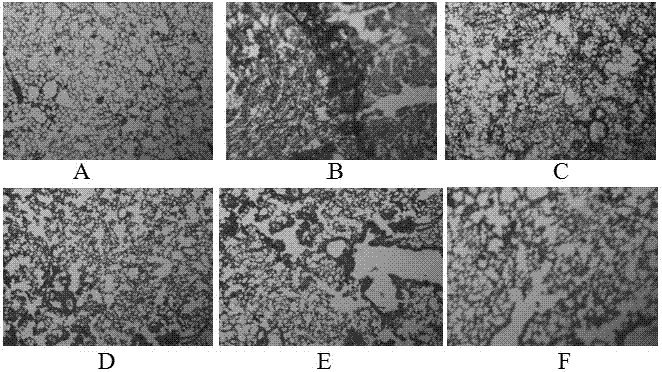
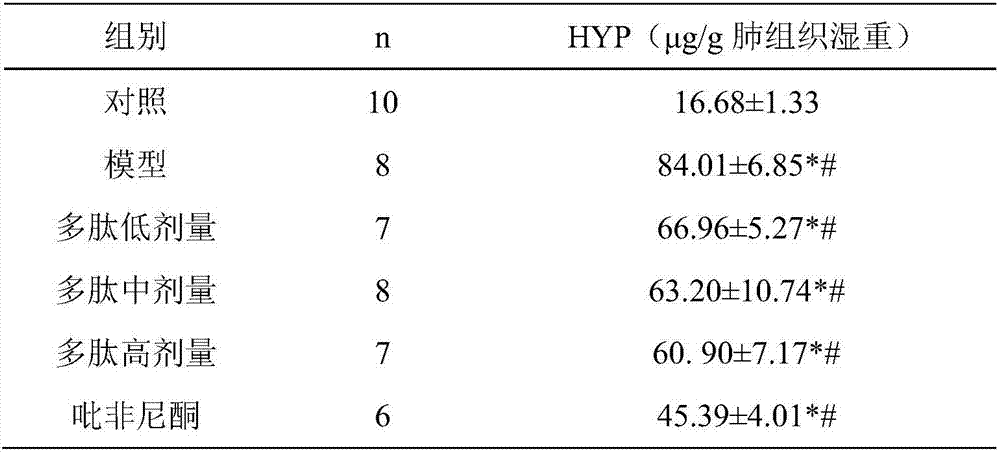
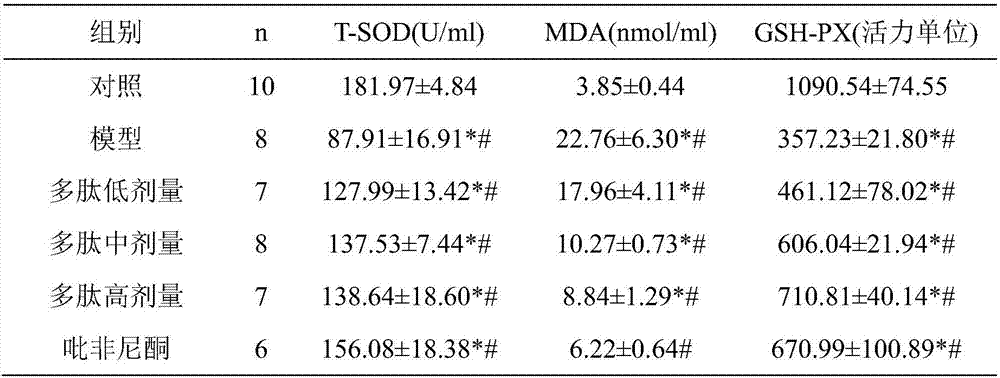
[0044] Embodiment 2 prevents and treats pulmonary fibrosis experiment
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com