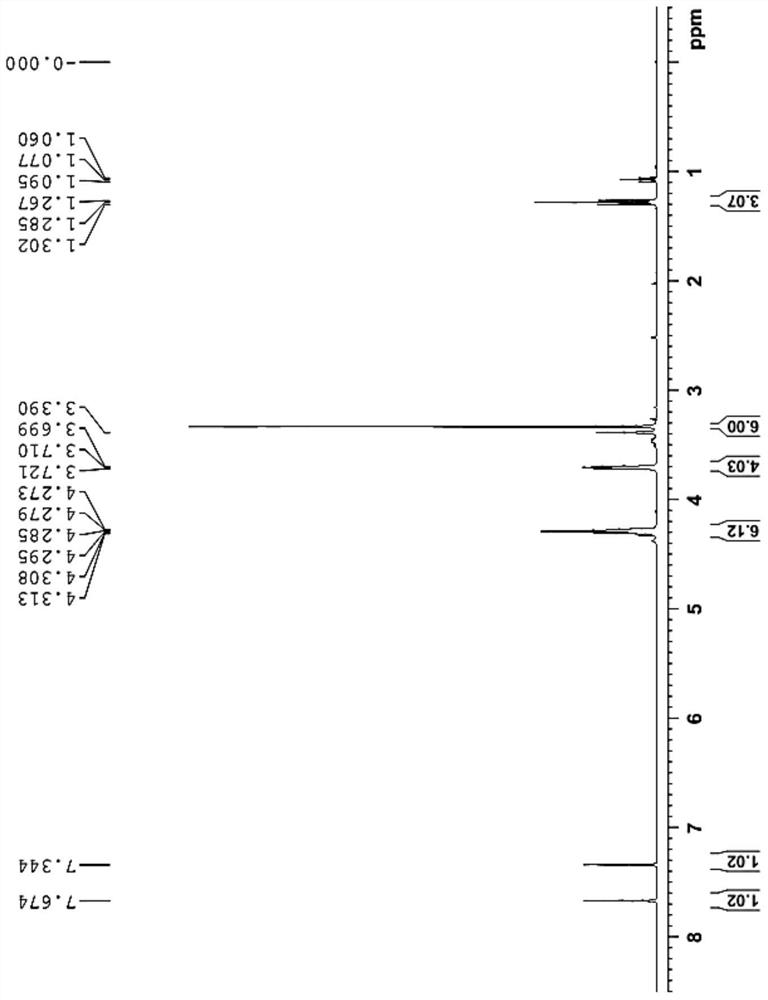
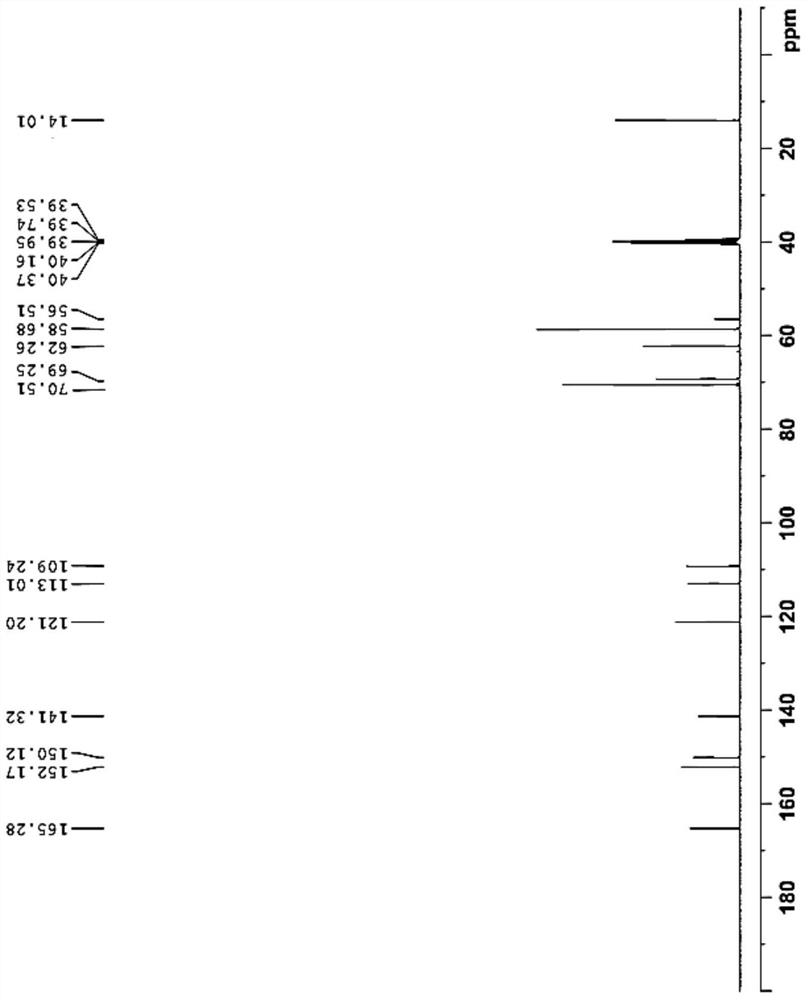
The preparation method of erlotinib hydrochloride key intermediate
A technology of acetic acid and nitric acid, applied in the field of medicinal chemistry, can solve the problems of reduced purity, increased side reactions, and increased waste liquid treatment costs.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0024] Add glacial acetic acid (70L), acetic anhydride (10L) and ethyl 3,4-bis(2-methoxyethoxy)benzoate (23kg, 77.1mol) into a 200L reactor, slowly add nitric acid ( 12L, 269.85mol). Another concentrated sulfuric acid (200ml, 3.85mol) was diluted in glacial acetic acid (2L). After the addition of nitric acid is completed, the diluted concentrated sulfuric acid solution in glacial acetic acid is slowly added dropwise to the reaction kettle. After the drop is complete, the temperature is raised to 40°C for 2 hours. After the reaction is complete, cool the reaction solution to 20°C, slowly pour it into a mixture of ice water (80L) and dichloromethane (80L) while stirring, separate the lower dichloromethane layer, and add dichloromethane to the upper water layer (80L) extracted once, the lower organic layer was released, and the organic phases were combined. The organic phase was washed successively with purified water (80 L), aqueous sodium bicarbonate (100 L), and saturated br...
example 2
[0027] Add glacial acetic acid (80L), acetic anhydride (15L) and ethyl 3,4-bis(2-methoxyethoxy)benzoate (27kg, 90.5mol) into a 200L reactor, slowly add nitric acid ( 14L, 316.75mol). Another concentrated sulfuric acid (10ml, 0.18mol) was diluted in glacial acetic acid (1L). After the addition of nitric acid is completed, the diluted concentrated sulfuric acid solution in glacial acetic acid is slowly added dropwise to the reaction kettle. After the drop is complete, the temperature is raised to 40°C for 2 hours. After the reaction is complete, cool the reaction solution to 20°C, slowly pour it into a mixture of ice water (80L) and dichloromethane (80L) while stirring, separate the lower dichloromethane layer, and add dichloromethane to the upper water layer (80L) extracted once, the lower organic layer was released, and the organic phases were combined. The organic phase was washed successively with purified water (80 L), aqueous sodium bicarbonate (100 L), and saturated bri...
example 3
[0029]Add glacial acetic acid (80L), acetic anhydride (15L) and ethyl 3,4-bis(2-methoxyethoxy)benzoate (25kg, 83.8mol) into a 200L reactor, and slowly add nitric acid ( 13 L, 293.3 mol). Another concentrated sulfuric acid (460ml, 8.4mol) was diluted in glacial acetic acid (1L). After the addition of nitric acid is completed, the diluted concentrated sulfuric acid solution in glacial acetic acid is slowly added dropwise to the reaction kettle. After the drop is complete, the temperature is raised to 40°C for 2 hours. After the reaction is complete, cool the reaction solution to 20°C, slowly pour it into a mixture of ice water (80L) and dichloromethane (80L) while stirring, separate the lower dichloromethane layer, and add dichloromethane to the upper water layer (80L) extracted once, the lower organic layer was released, and the organic phases were combined. The organic phase was washed successively with purified water (80 L), aqueous sodium bicarbonate (100 L), and saturated...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com