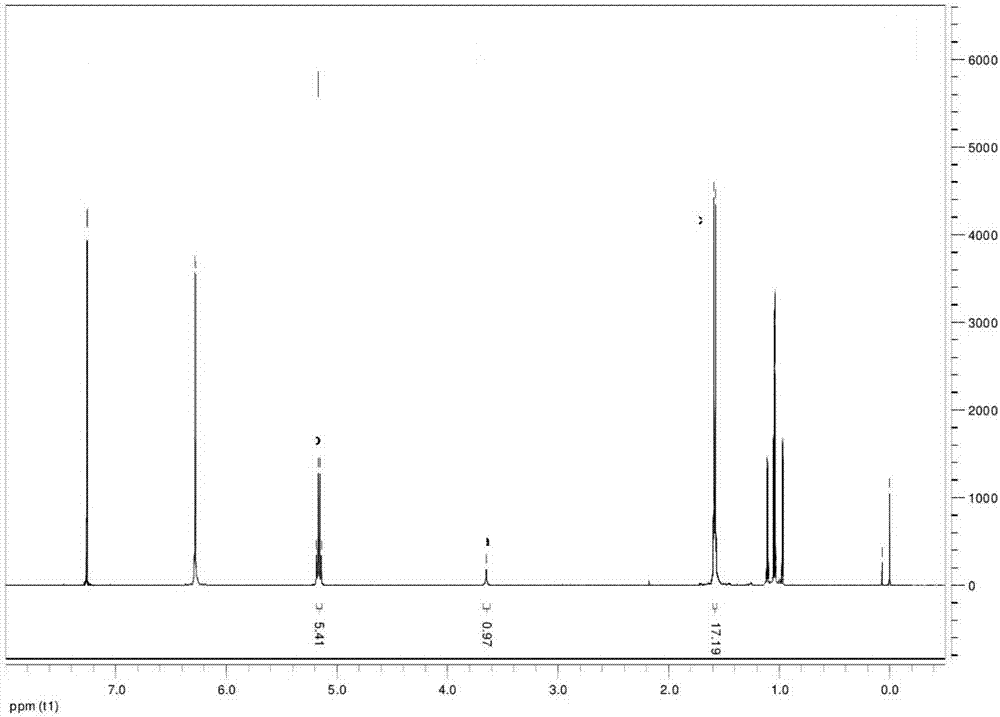
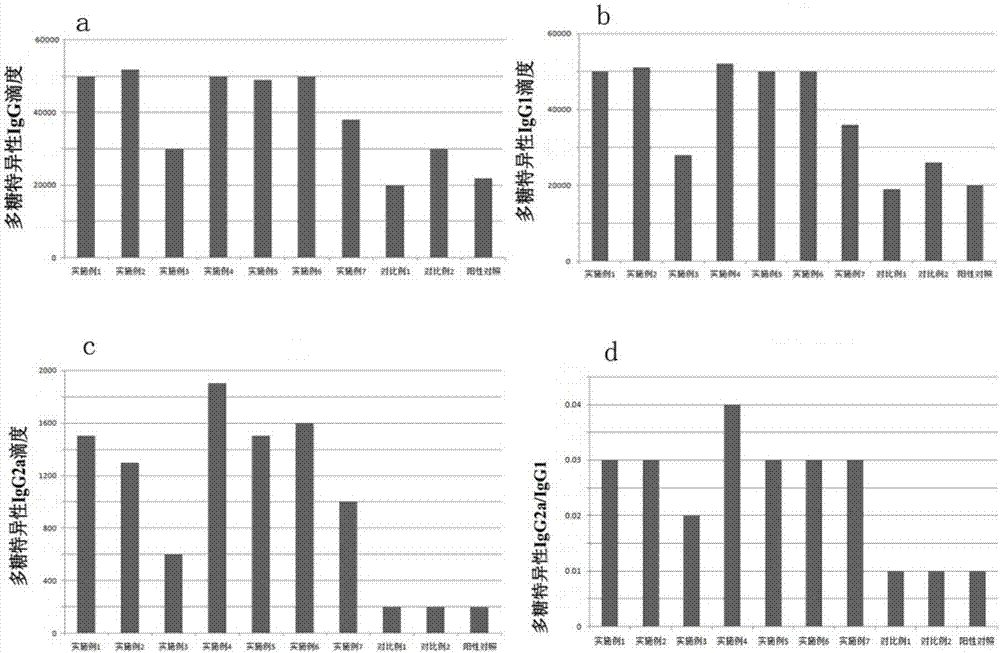
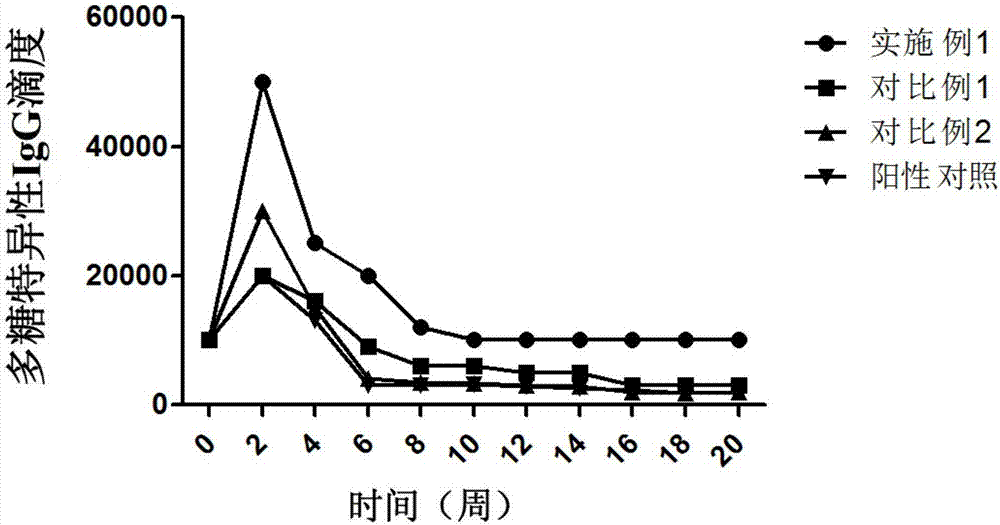
Rotavirus polysaccharide-protein conjugate vaccine and preparation method thereof
A protein-conjugated vaccine and rotavirus technology, applied in the rotavirus polysaccharide-protein conjugate vaccine vaccine and its preparation, in the field of vaccines, can solve the problem that preventive polysaccharide and/or protein vaccines have not been reported and are not easy to enhance immunity , inability to induce immune memory effects, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0109] 1. Preparation of Magnetic Nanospheres
[0110] 1. Take 2.24g FeSO 4 -7H 2 O and 3.24 g FeCl 3 -6H 2 O dissolved in 10 mL and 15 mL of dddH 2 In O, mix well after dissolving, add 100mL of dddH filtered to remove oxygen 2 O;
[0111] 2. In N 2 Stir for 5 minutes under the protection of the solution, add 50mL1mol / L NaOH solution at one time, adjust the pH of the solution to 9-10, increase the stirring speed to 200-250r / min, and continue stirring for 30min;
[0112] 3. Transfer the reaction vessel to a 65-70°C water bath and continue to 2 Stirring and aging under the protection of 30min;
[0113] 4. After the reaction, set the volume to 100mL, and observe the synthesis of magnetic particles under a microscope;
[0114] 5. Dissolve 400mgPLGA in 10mLEA solvent as the oil phase (O), add 3mL of the above magnetic particle solution as the inner water phase (W 1 ), using an ultrasonic cell breaker (120W, 60s) to carry out colostrum in an ice-water bath to prepare colos...
Embodiment 2
[0158] The difference between this example and Example 1 is that the carrier protein of the rotavirus polysaccharide-protein conjugate vaccine is rotavirus carrier protein and tetanus toxoid carrier protein.
[0159] The composition analysis results of the prepared rotavirus polysaccharide-protein conjugate vaccine are consistent with the results obtained in Example 1 within the theoretical error range.
Embodiment 3
[0161] The difference between this example and Example 1 is that the carrier protein of the rotavirus polysaccharide-protein conjugate vaccine is rotavirus carrier protein and Haemophilus influenzae surface protein HiD.
[0162] The composition analysis results of the prepared rotavirus polysaccharide-protein conjugate vaccine are consistent with the results obtained in Example 1 within the theoretical error range.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com