Synthesis method of (R) 3-amino-2-oxoindole derivative
A compound, the technology of dioxane, applied in the field of synthesis of chiral-3-amino-2-oxidole derivatives, can solve problems such as harmfulness and ineffectiveness, achieve mild reaction conditions, broaden the scope of application, and be suitable for industrialization The effect of mass production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
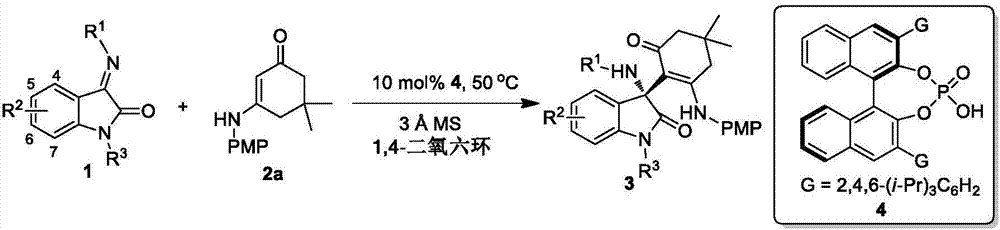
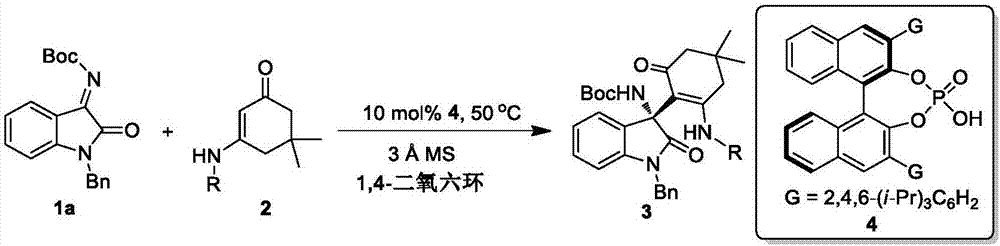
[0033] Example 1: Add 0.2 mmol of the compound of formula 1a and 0.1 mmol of the compound of formula 2a in 1 ml of 1,4-dioxane as reactants, 100 mg Molecular sieve is used as an additive, 0.01 millimole of chiral phosphoric acid (that is, the compound of formula 4 in this patent) is used as a catalyst, and the corresponding (R) 3-amino-2-oxindole derivative is obtained by reacting at 50° C. for 48 hours.
[0034]
Embodiment 1-11
[0036] Attached below figure 1 And attached figure 2 And embodiment further describes the present invention:
[0037] Reaction raw materials, reaction conditions and productive rate are as shown in table 1:
[0038] Table 1*
[0039]
[0040]
[0041] * 0.2 mmol of compound of formula 1 and 0.1 mmol of compound of formula 2a were used as reactants, 0.01 mmol of compound of formula 4 was used as catalyst, and 1 ml of 1,4-dioxane was used as solvent.
[0042] In table 1, the isatin imine of embodiment 1~9 reaction is the isatin imine of 4-7 position substitution, and the isatin imine of embodiment 10 reaction is the isatin imine of ester group protection, and embodiment 11 reaction The isatinimines are different N-benzyl substituted isatinimines.
Embodiment 12-11
[0044] Reaction raw materials, reaction conditions and productive rate are as shown in table 2:
[0045] Table 2*
[0046]
[0047] * 0.2 mmol of isatinimine 1a and 0.1 mmol of enaminone 2 were used as reactants, and 0.01 mmol of chiral phosphoric acid 4 was used as catalyst.
[0048] In Table 2, the enaminones reacted in Examples 12-21 are the enaminones of damidones derived from various anilines, and the enaminones reacted in Example 22 are enaminones of dammidones derived from naphthylamine.
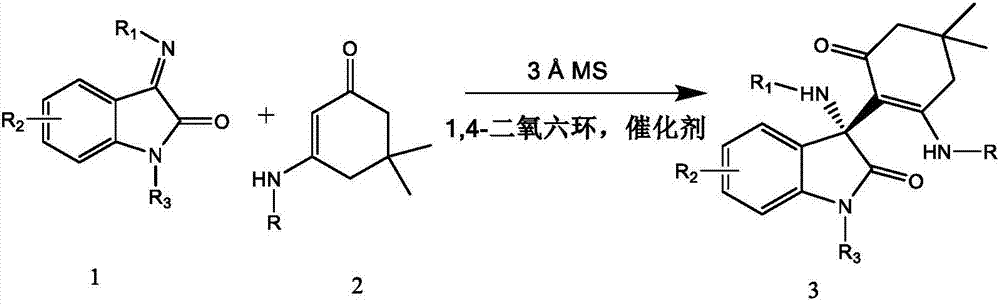
[0049] As can be seen from Table 1 and Table 2, the method of the present invention can not only realize the diversity and complexity of product molecules in one step, obtain high enantioselectivity, high atom economy, environmental friendliness, and wide application range, but also raw materials are easy to obtain, The operation is simple and safe, the reaction conditions are mild, the reaction time is short, the post-treatment is simple, and the product structure is diversified, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com