Axially chiral aryl indole compound and synthesis method thereof
An aryl indole compound, a technology for a synthesis method, applied in the directions of organic chemistry, organic active ingredients, organic chemistry, etc., can solve problems such as limitation, achieve mild reaction conditions, high yield, and be suitable for industrialized large-scale production.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
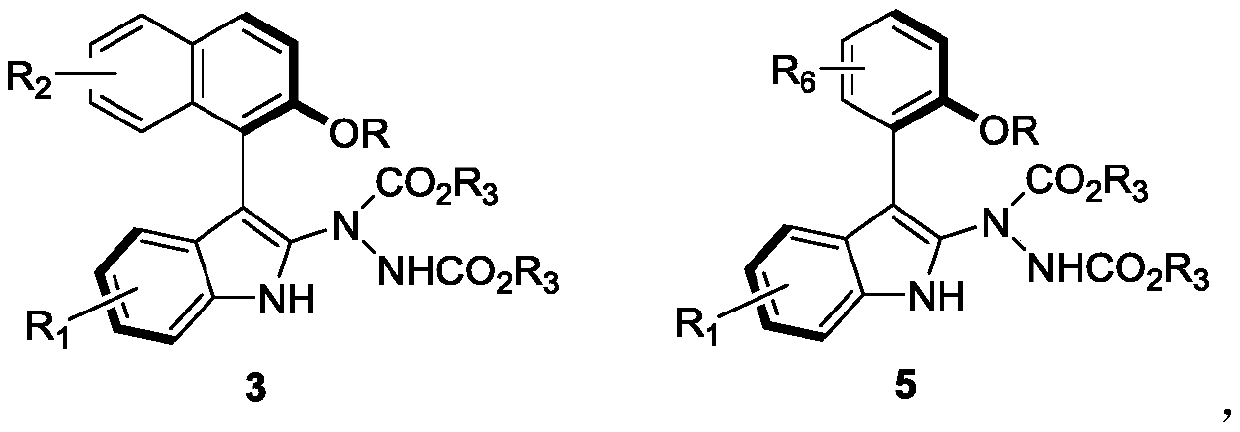
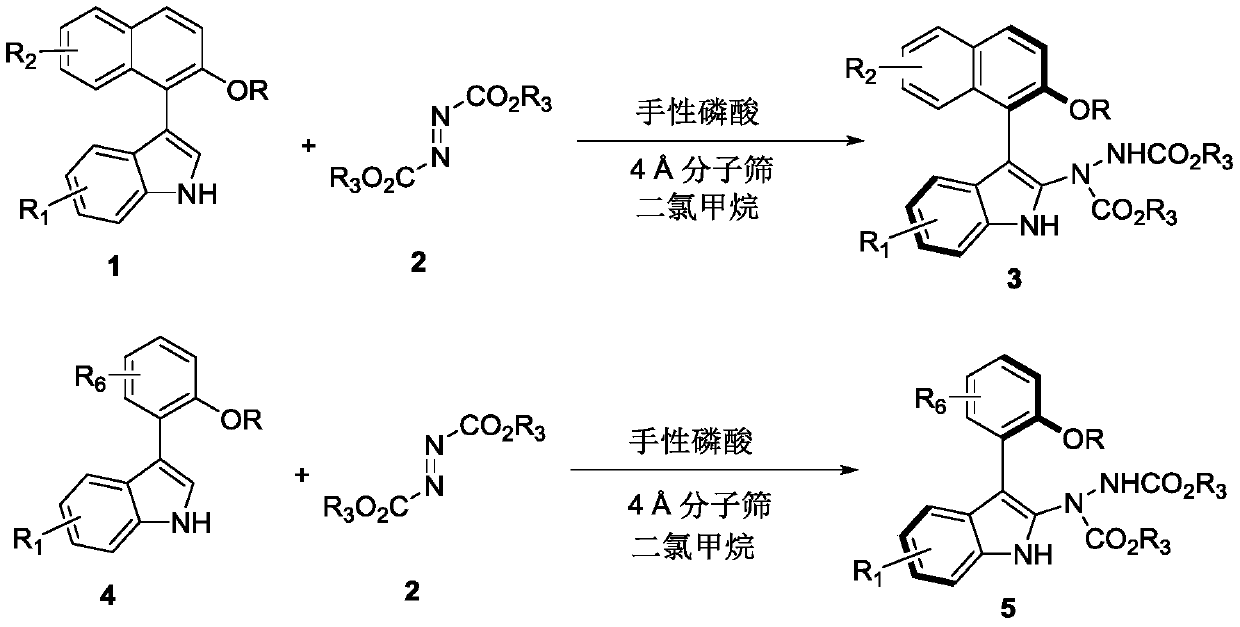
[0031] Embodiment 1: in 1 milliliter of dichloromethane, add the formula 1c compound of 0.1 millimole and the formula 2b compound of 0.3 millimole as reactant, 100 milligrams Molecular sieves are used as additives, 0.01 mmol of chiral phosphoric acid (i.e. the compound of formula 61) is used as a catalyst, reacted at 25 ° C for 48 hours, TLC traced the reaction to the end, and filtered to remove Molecular sieves, wash the filter cake with ethyl acetate, and separate the obtained filtrate through silica gel column chromatography (the eluent is a mixed solution with a volume ratio of petroleum ether and ethyl acetate of 10:1) after concentration to obtain the axial chiral indole Inole-naphthalene 3cb, white solid.
[0032] The structural characterization data of product 3cb in Example 1 are as follows:
[0033] m.p.86.6-87.6°C; [α] D 20 = -44.7(c 0.81, acetone); 1 H NMR (400MHz, DMSO-d 6 )δ11.48(s,1H),10.05(s,1H),8.15(d,J=9.1Hz,1H),8.08(d,J=8.2Hz,1H),7.62–7.55(m,2H), 7.5...
Embodiment 18
[0041] Embodiment 18: in 4 milliliters of dichloromethane, add 0.1 millimole formula 1a compound and 0.12 millimole formula 2a compound as reactant, 100 milligrams Molecular sieves are used as additives, 0.01 mmol of chiral phosphoric acid (i.e. the compound of formula 61) is used as a catalyst, react at 25°C for 5 hours, TLC tracks the reaction to the end, and removes by filtration Molecular sieves, wash the filter cake with ethyl acetate, and separate the obtained filtrate through silica gel column chromatography (the eluent is a mixed solution with a volume ratio of petroleum ether and ethyl acetate of 10:1) after concentration to obtain the axial chiral indole Inole-naphthalene 3aa, white solid.
[0042] The structural characterization data of product 3aa in Example 18 are as follows:
[0043] m.p.85.1-85.8℃; [α] D 20 = -13.7(c 1.85, acetone); 1 H NMR (400MHz, CDCl 3 )δ 1 HNMR (400MHz, CDCl 3 )δ9.36(s,1H),7.93(d,J=9.0Hz,1H),7.87(d,J=7.7Hz,1H),7.72–7.55(m,2H),7.50(...
Embodiment 25
[0050] Example 25: Add 0.1 mmol of the compound of formula 4u and 0.3 mmol of the compound of formula 2b in 1 ml of dichloromethane as a reactant, 100 mg Molecular sieves are used as additives, 0.01 mmol of chiral phosphoric acid (i.e. the compound of formula 61) is used as a catalyst, reacted at 25 ° C for 48 hours, TLC traced the reaction to the end, and filtered to remove Molecular sieves, wash the filter cake with ethyl acetate, and separate the obtained filtrate through silica gel column chromatography (the eluent is a mixed solution with a volume ratio of petroleum ether and ethyl acetate of 10:1) after concentration to obtain the axial chiral indole Indole-naphthalene 5ub, white solid, yield 86%.
[0051] The structural characterization data of product 5ub in Example 25 are as follows:
[0052] m.p.113.2-113.8°C; [α] D 20 = -5.1(c 0.71, acetone); 1 H NMR (400MHz, DMSO-d 6 )δ11.48(s,1H),10.16(s,1H),7.46(d,J=8.1Hz,1H),7.40–7.20(m,10H),7.19–7.09(m,2H),6.98(d ,J=4.9...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com