1-Aromatic aldoxime uracil and its preparation method
A technology of oxime uracil and aromatic aldoxime, which is applied in the field of 1-aryl aldoxime uracil and its preparation, can solve problems such as toxicity and drug resistance, achieve high feasibility, inhibit thymidine phosphorylase and anti-tumor activity , the effect of reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
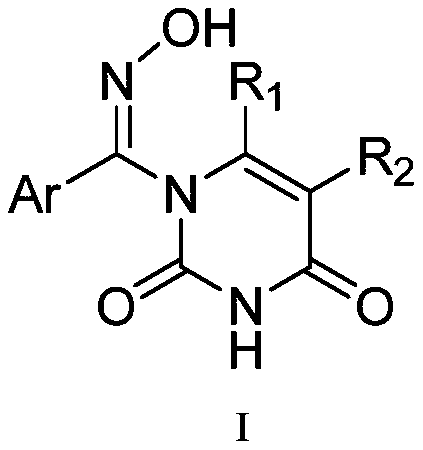
[0022] Embodiment 1: A kind of 1-aromatic aldoxime uracil has the following structure I:
[0023]
[0024] Wherein, R1 is methyl or hydrogen, R2 is chlorine, iodine, carboxyl or hydrogen, Ar is alkylphenyl, halogenated phenyl, alkoxyphenyl, thienyl, furyl.
[0025] The 1-aromatic aldoxime uracil has a structural formula:
[0026]
[0027] The preparation method of 1-aromatic aldoxime uracil, its synthetic reaction formula is:
[0028]
[0029] R 1 =CH 3 , H R 2 = Cl, I, COOH, H
[0030] Concrete preparation steps are as follows:
[0031] (1) Dissolve 0.02mol of aromatic aldehyde in 25mL of methanol and stir at room temperature. After the aromatic aldehyde is completely dissolved, add 0.02mol of hydroxylamine hydrochloride and 0.022mol of potassium carbonate into methanol and react for 3 hours. After the reaction is complete, extract the reaction solution. Then wash with saturated sodium bicarbonate solution, dry with anhydrous sodium sulfate, and recover metha...
Embodiment 2
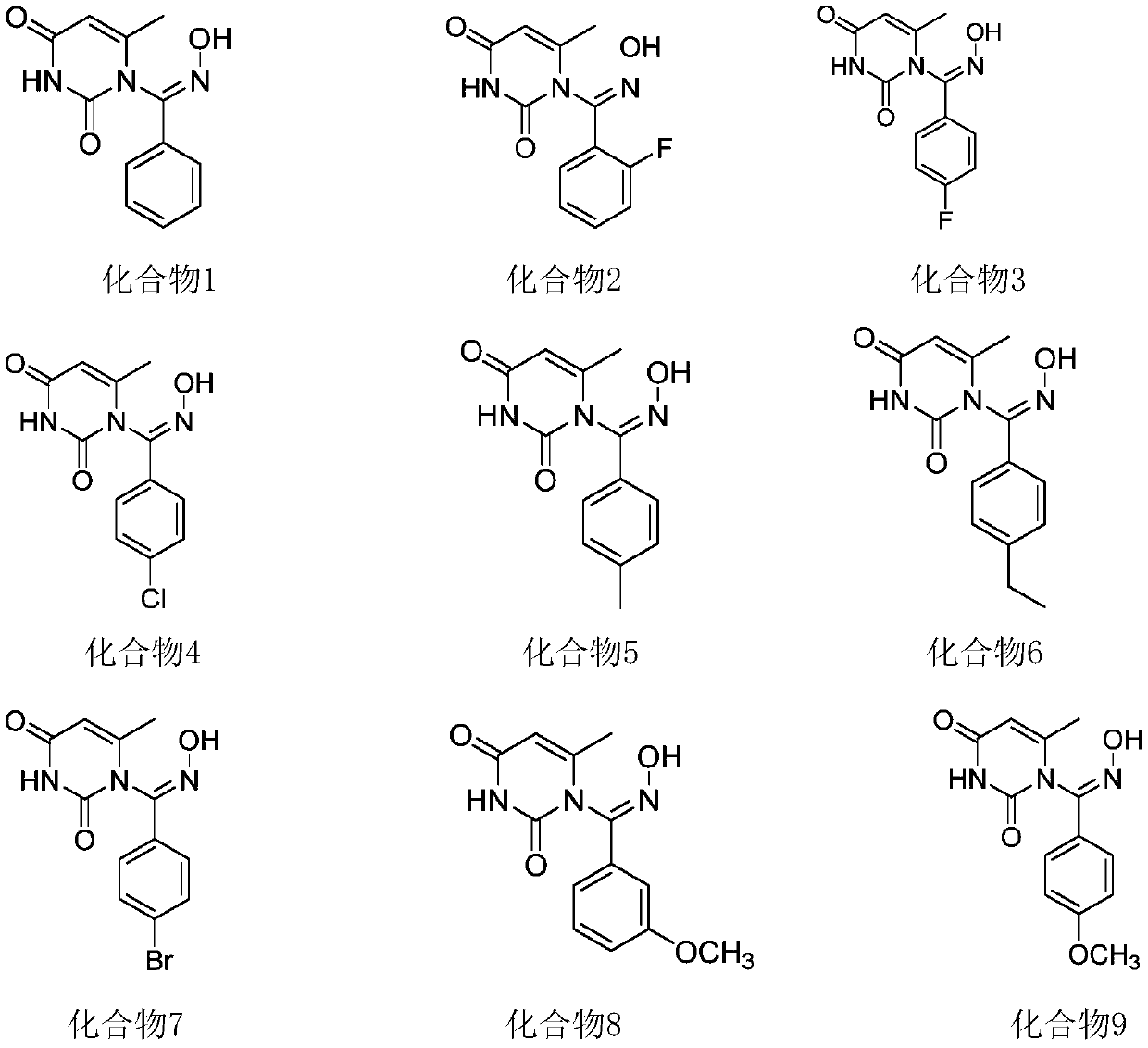
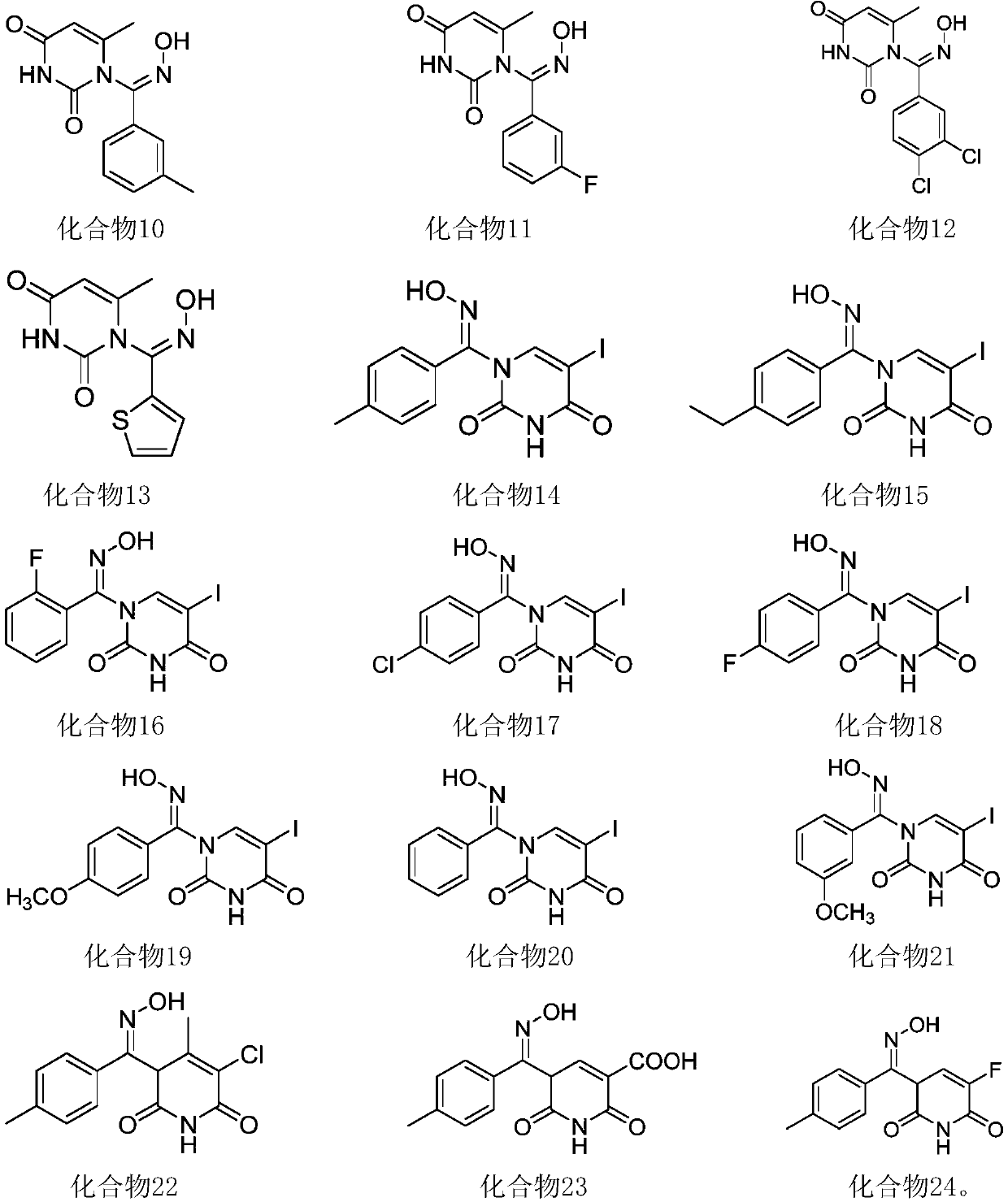
[0044] Embodiment 2: a kind of 1-aromatic aldoxime uracil, its structural formula is
[0045]
[0046] (Z)-1-((2-fluorophenyl)(oximino)methyl)-6-methylpyrimidine-2,4-(1H,3H)-dione (Compound 2).
[0047] White solid; M.p.248-249°C; IR(KBr,ν max ,cm -1 ):3440,2919,1695,1432,1302,1179,989,811,512; 1 H NMR (600MHz, DMSO-d 6 )(δ,ppm):12.57(s,1H,NH),11.50(s,1H,OH),7.52-7.47(m,J=7.6Hz,3H,ArH),7.46-7.33(m,1H,ArH ),5.73(s,1H,CH),1.89(s,3H,CH 3 ); 13 CNMR (150MHz, DMSO-d 6 )(δ,ppm):163.4,162.1,152.3,149.8,142.5(d, 3 J C–F =3Hz), 134.6(d, 3 J C–F =7.5Hz), 131.7(d, 3 J C–F =7.5Hz), 121.7(d, 3 J C–F =1.5Hz), 117.6(d, 2 J C–F =21Hz), 112.3(d, 2 J C–F =24Hz), 101.8, 18.3; HRMS (ESI-TOF, [M+H] + ): m / z calcd for C 12 h 10 FN 3 o 3 , 264.0779; found, 264.0776.
Embodiment 3
[0048] Embodiment 3: a kind of 1-aromatic aldoxime uracil, its structural formula is
[0049]
[0050] (Z)-1-((4-fluorophenyl)(oximino)methyl)-6-methylpyrimidine-2,4-(1H,3H)-dione (compound 3).
[0051] White solid; M.p.234-235℃; IR(KBr,νmax,cm-1):3419,2921,1706,1513,1459,1159,841,605,538;1H NMR(500MHz,DMSO-d6)(δ,ppm):12.36 (s,1H,NH),11.48(s,1H,OH),7.70-7.67(m,J=8.5Hz,2H,ArH),7.31-7.28((m,J=7Hz,2H,ArH),5.72 (s,1H,CH),1.89(s,3H,CH3); 13 C NMR (125MHz, DMSO-d 6 )(δ,ppm):163.4,162.7,152.4,149.8,142.5,128.7(d, 3 J C–F =2.5Hz), 128.0(d, 3 J C–F =8.8Hz), 116.7, 116.5, 101.6, 18.3; HRMS (ESI-TOF, [M+H] + ): m / z calcd for C 12 h 10 FN 3 o 3 , 264.0779; found, 264.0776.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com