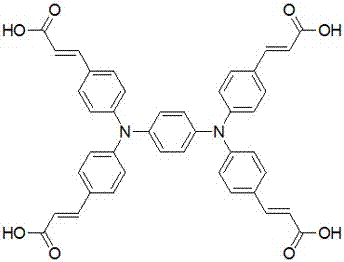
Synthesizing method for tetracarboxylic acid N,N,N',N'-tetra (4-carboxyl vinyl phenyl)-1,4-phenylenediamine
A technology of vinyl phenyl and tetracarboxylic acid, applied in the field of synthesis of tetracarboxylic acid N,N,N',N'-tetra-1,4-phenylenediamine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0022] of the present invention N , N , N ', N The synthetic method of '-tetra(4-carboxyvinylphenyl)-1,4-phenylenediamine, the synthetic steps are:
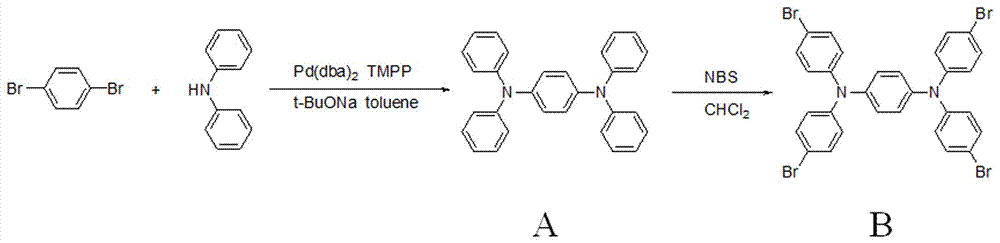
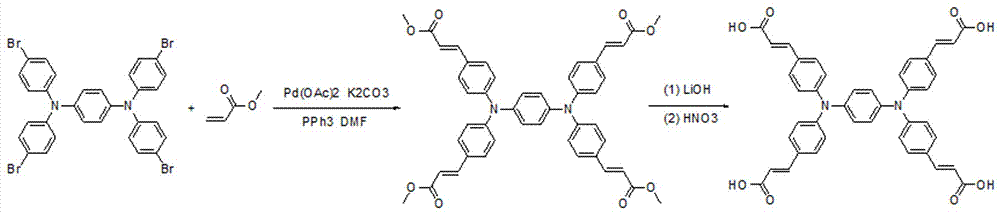
[0023] Using 1,4-dibromobenzene and diphenylamine as raw materials, it is produced by Buchwald-Hartwig aryl amination reaction N,N,N',N' -Tetraphenyl-1,4-phenylenediamine (A), produced by the bromination reaction of A and NBS N,N,N',N' -Tetrakis(4-bromophenyl)-1,4-phenylenediamine (B), the synthetic route is as follows figure 2 shown. B reacts with methyl acrylate to generate by Heck reaction N,N,N',N' -Tetrakis(4-methoxycarbonylvinylphenyl)-1,4-phenylenediamine (C), C is finally hydrolyzed to obtain the target compound N,N,N',N' -Tetrakis(4-carboxyvinylphenyl)-1,4-phenylenediamine (D), the synthetic route is as follows image 3 shown.
specific Embodiment
[0025] (i) if figure 2 Shown: weigh 1,4-dibromobenzene (2.9 g, 12.3 mmol), diphenylamine (5.0 g, 29.6 mmol), bis(dibenzylidene ketone) palladium (0.04 g, 0.07 mmol), tert-butanol Sodium (2.9 g, 30.2 mmol) was added to a 250 mL three-neck flask, 50 mL of toluene was added, and 3.5 mL of tris-(2-tolyl)phosphine was added quickly after argon gas flowed for 15 min, and the reaction was carried out in an oil bath at 90°C under the protection of argon After 12 h, the toluene was distilled off at the end of the reaction, diluted with 30 mL of water, extracted with dichloromethane (30 mL×3), the organic layer was collected, and anhydrous Na 2 SO 3 Dry, filter, and distill off the solvent to obtain the reaction crude product, which is purified by silica gel column chromatography (eluent: petroleum ether / ethyl acetate = 20:1) after drying to obtain white powder A (3.04 g, 7.4 mmol ). The rate is 60%. Melting point: 197~198°C.
[0026] Compound A NMR 1 H NMR, 13 C NMR and high-res...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap