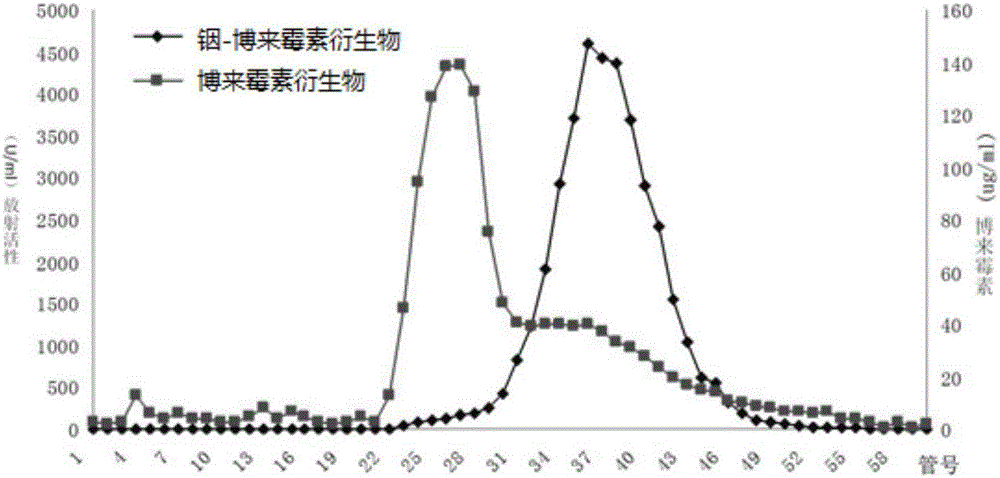
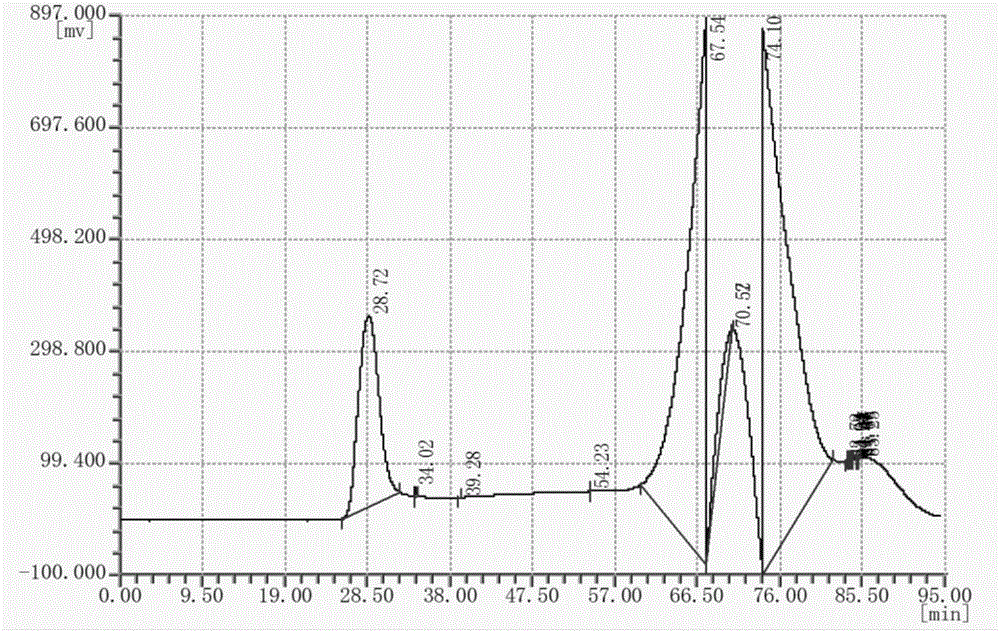
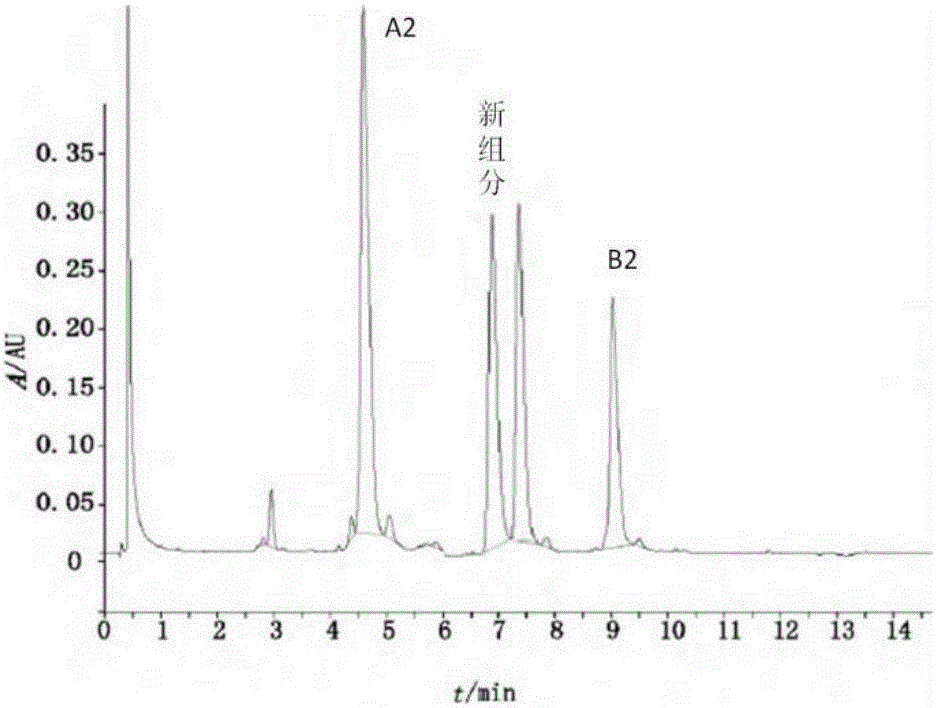
Bleomycin derivative and preparation method and application thereof
A technology of bleomycin and its derivatives, which is applied in the field of bleomycin derivatives and its preparation, can solve the problems of short half-life, poor enrichment and toxicity of bleomycin, and achieve little harm and strong accumulation in the body. Effect of reducing binding force and adverse reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0069] The preparation method of bleomycin derivative comprises the steps:
[0070] (1) Strain activation: prepare Gaoshi No. 1 medium: dissolve 10 g of soluble starch, 0.5 g of potassium chloride, 1 g of potassium dihydrogen phosphate, 0.5 g of magnesium sulfate, 2 g of sodium nitrate, and 2 g of ferrous sulfate in 1 liter of water , inoculate Streptomyces verticillium into the culture medium, culture at 28°C for 7 days; harvest monospore suspension, concentration 10 8 a / ml;
[0071] (2) Secondary fermentation: preparation of fermentation medium: glucose 10g, soybean cake powder 30g, corn steep liquor 30g, industrial starch 30g, maltose 20g, sodium chloride 3g, dipotassium hydrogen phosphate 1g, zinc sulfate 0.5g, soybean peptone 5g, dissolved in 1000ml water, inoculated Streptomyces verticillium activated in step (1) into the culture medium, cultured at 28°C for 5 days; Molecule, dropwise, control the highest concentration to 3μm / l;
[0072] (3) Tertiary fermentation: pre...
Embodiment 2
[0077] 111 The preparation method of the chelate of indium-bleomycin derivative: get N 2 Add 1mg of dried bleomycin derivative dry powder to 80μl 111 InCl 3 In the solution, use 1M NaCl solution to adjust the ionic strength at 250-300mOsm / kg·H 2 Between 0 and 20-30°C, stir gently for more than 30 minutes to dissolve the bleomycin derivative to obtain a mixed solution. During the stirring process, the pH value of the control system was 2.0.
[0078] Use macroporous adsorption resin X-5 to desalt the resulting mixed solution, and put the desalted clear liquid on CM-SephadexC-25[NH 4 + ] chromatography column with 0.1M NH 4 Get it by repeated elution with Cl-HCl solution, measure the osmotic pressure of the eluent, and adjust the osmotic pressure to 250-350mOsm / kg·H 2 O range is available.
Embodiment 3
[0080] A kind of injection, containing 1mg / dose of embodiment 1 in the injection 111 Chelates of indium-bleomycin derivatives.
[0081] After testing, the injection 111 The radioactivity of indium is 5mCi / dose.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap