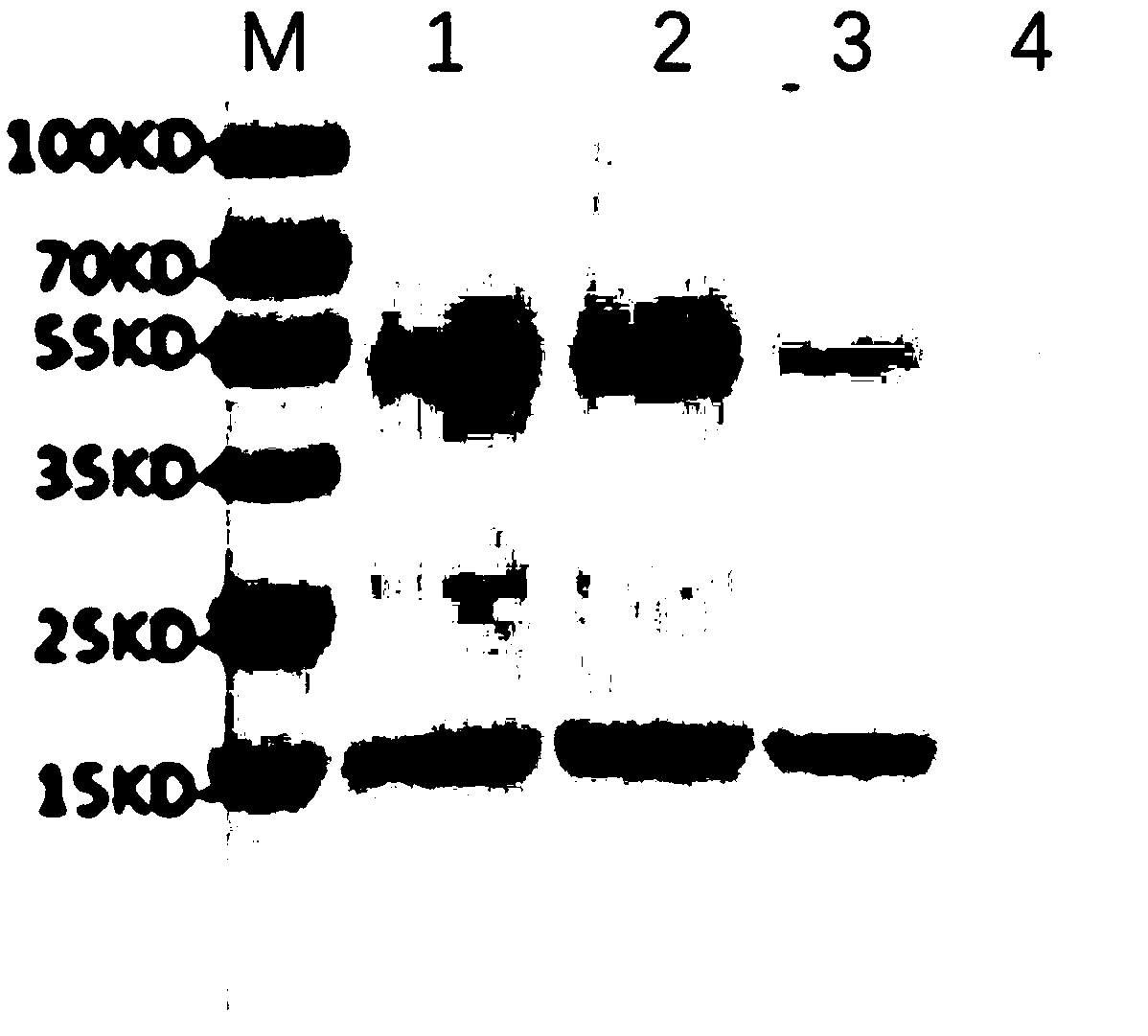A kind of Escherichia coli lyase and its preparation method and application
A technology of Escherichia coli and lyase, applied in the field of genetic engineering, can solve the problems of narrow phage lysis spectrum and variation, and achieve the effects of no toxic side effects, safe toxic side effects, and high prevention and control effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Example 1 Cloning of recombinant chimeric lyase LysBM1 gene, construction of expression vector and expression strain
[0036] (a) Design the chimeric lyase LysBM1 gene according to the structural characteristics of E. coli cell wall and the lyase gene sequence of E. coli virulence phage JS25 isolated by our laboratory, and its nucleotide sequence is shown in Seq ID NO.1
[0037](ATGCARATHTTYAAYATGMGNACNAAYGAYGARGGNGTNYTNGGNYTNMGNYTNACNYTNTAYAARGAYACNMGNGGNTTYTGGACNATHGGNGCNGGNATHTGGACNGTNTGYGCNAAYCCNGTNTGYGCNGGNGCNYTNGAYMGNATGATHGGNMGNAARTGYAAYCCNACNGTNTGYYTNYTNAARGCNMGNAARCARTTYAAYAARAARGTNAAYAARGCNGGNMGNGGNGCNATHGCNGGNAAYGCNWSNGTNAARCCNGTNTAYAARWSNYTNAARGCNGGNYTNGCNWSNGCNYTNGTNAAYATGGCNTTYMGNMGNGCNWSNTTYCCNTAYAAYGTNGCNTTYMGNWSNYTNMGNYTNYTNAARAARCARAARMGNTGGMGNAAYYTNAAYGCNGGNAARGCNTGYMGNAARTGGTAYMGNCARACNCCNAAYMGNGCNAARMGNGTNATHAARACNTGGGCNGGNGGNAARGTNWSNMGNMGNGAYATHGAR)。
[0038] (b) Construction of the recombinant Pichia expression vector of the lyase: according to th...
Embodiment 2
[0041] Example 2 Induced expression and purification of chimeric lyase LysBM1 protein
[0042] Induced expression: pick positive clones and inoculate them in 50ml BMGY medium (yeast extract 1%, tryptone 2%, potassium phosphate buffer (pH6.0) 100mmol / L, YNB 1.34%, biotin (4×10 -5 )% glycerol 1%), 30°C, 250rpm shaking culture until OD600 is between 4-6, take it out, centrifuge at 1500g for 5min, collect yeast cells, and then resuspend in 1 / 5 volume of BMMY medium (yeast extract 1%, tryptone 2%, potassium phosphate buffer (pH6.0) 100mmol / L, YNB 1.34%, biotin (4×10 -5 )%, methanol 3%) to continue culturing in the Erlenmeyer flask, after 24 hours, add methanol every day to a final concentration of 2.5-3%. The culture was continued for 6-10 days, and the supernatant was collected by centrifugation.
[0043] The supernatant of chimeric lyase LysBM1 yeast expression was directly taken for SDS-PAGE, and electrophoresis showed that the target protein was expressed, and the protein amo...
Embodiment 3
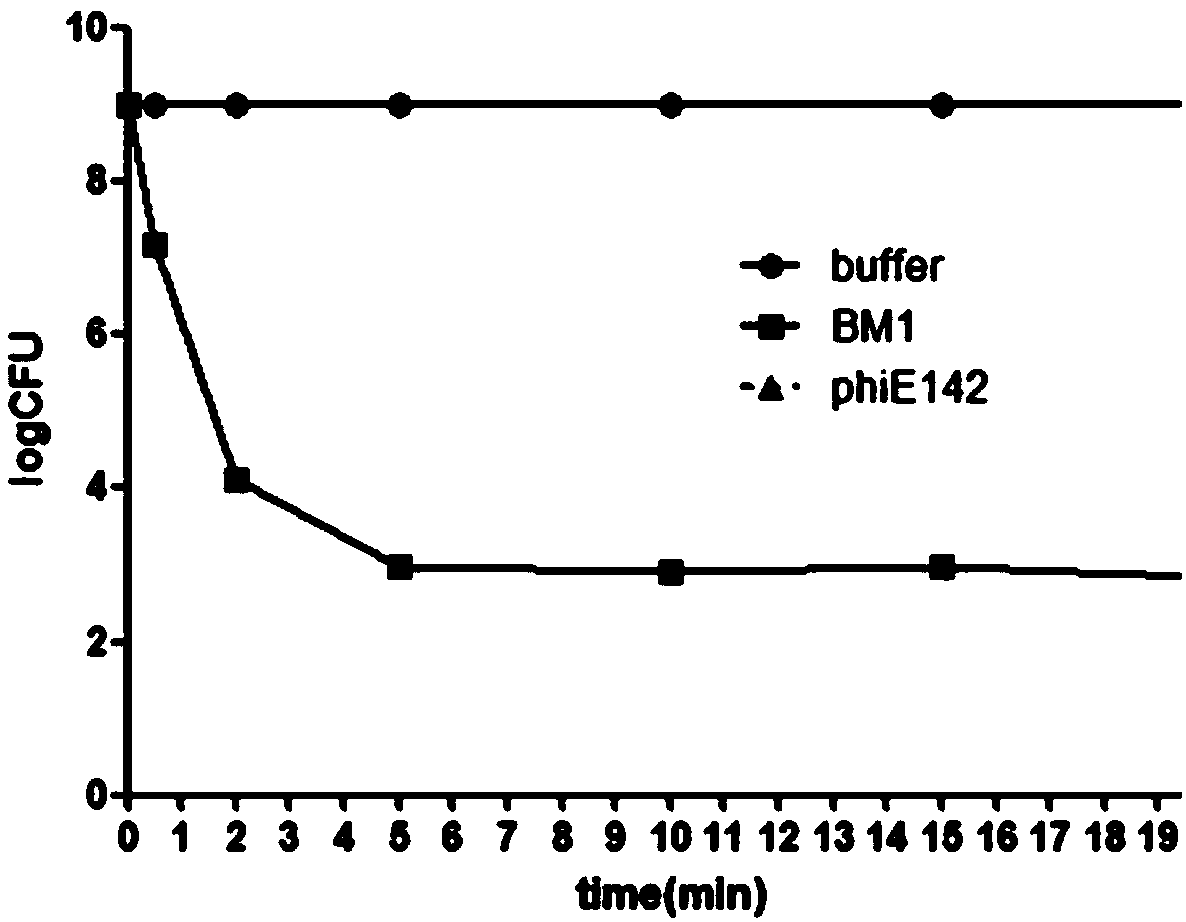
[0044] Example 3 Analysis of the bactericidal spectrum of the chimeric lyase LysBM1
[0045] The lyase with a final concentration of 10 μg / ml was selected to act on Escherichia coli ATCC25922 and 22 strains of Escherichia coli isolated clinically, as well as Staphylococcus aureus ATCC25923, Salmonella CMCC (B) 50115, and Pseudomonas aeruginosa ATCC27853 for detection The ability to form an inhibition zone on the plate. As shown in Table 1, the cleavage spectrum of the lyase LysBM1 is much larger than that of the phage V_EcoM_BM1, and it can lyse Escherichia coli in a broad spectrum.
[0046] Table 1 Comparison of cleavage profiles of lyase LysBM1 and phage V_EcoM_BM1
[0047]
[0048] Among them, "-" means no lysing; "+" means incomplete lysing, and 12 hours later, there are colonies growing in the inhibition plaque; "++" means complete lysing.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com