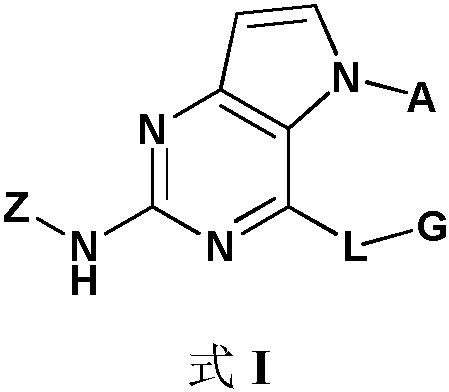
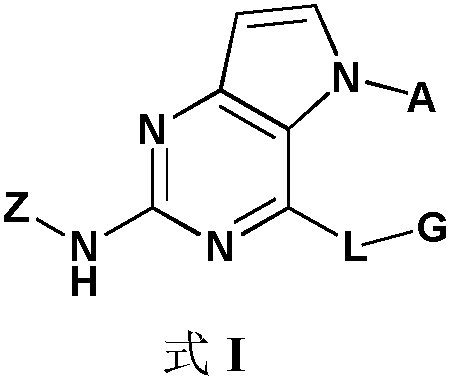
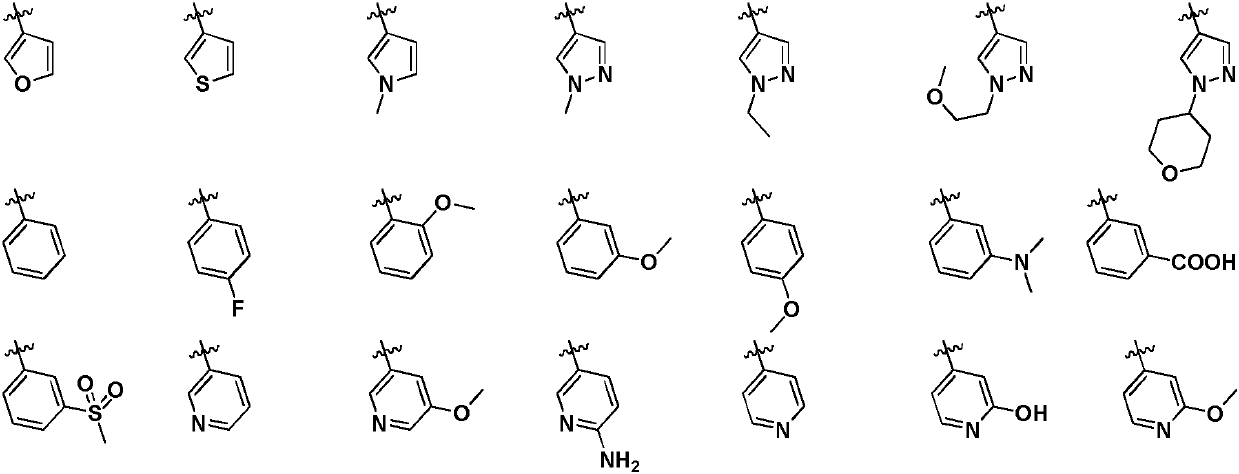
irak4 inhibitor and its application
A technology of inhibitors and alkyl groups, applied in the field of medicine, can solve problems such as partial blockage of signal transduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0190] Preparation of compounds of the present invention
[0191] The following reaction schemes illustrate the preparation of the compounds of the present invention.
[0192] Those skilled in the art will understand that in the description herein, combinations of substituents are permissible only if such combinations result in stable compounds.
[0193] Those skilled in the art will also understand that in the methods described below, intermediate compound functional groups may need to be protected by an appropriate protecting group "PG". Such functional groups include hydroxyl, amino, mercapto and carboxyl. Suitable hydroxy protecting groups include trialkylsilyl or diarylalkylsilyl groups (eg tert-butyldimethylsilyl, tert-butyldiphenylsilyl or trimethylsilyl) , tetrahydropyranyl, benzyl, etc. Suitable protecting groups for amino, amidino and guanidino include tert-butoxycarbonyl, benzyloxycarbonyl and the like. Suitable protecting groups for mercapto include -C(=O)-R" ...
Embodiment 1
[0216] trans-4-[2-(3-methylisothiazol-5-ylamino)-5-(1-methyl-1H-pyrazol-4-yl)-5H-pyrrole[3,2-d] Preparation of pyrimidin-4-ylamino]cyclohexanol
[0217]
[0218] The first step: the preparation of 2,4-dichloro-5-(1-methyl-1H-pyrazol-4-yl)-5H-pyrrole[3,2-d]pyrimidine
[0219]
[0220] 2,4-dichloro-5H-pyrrole[3,2-d]pyrimidine (376mg, 2mmol), 1-methyl-4-(4,4,5,5-tetramethyl-1,3,2 -Dioxaborolan-2-yl)-1H-pyrazole (832 mg, 4 mmol), anhydrous copper acetate (363 mg, 2 mmol) and pyridine (158 mg, 2 mmol) were added to dimethylsulfoxide (10 mL) . The reaction solution was heated to 110°C and stirred overnight. The reaction solution was naturally cooled to room temperature, added ethyl acetate (50mL), filtered to remove insoluble matter, the filtrate was washed with water (40mL×2), dried over anhydrous sodium sulfate, concentrated under reduced pressure, and washed with n-hexane / ethyl acetate (2 / 1 , 30mL) of the mixed solution was beaten and filtered to obtain 150mg of light y...
Embodiment 45
[0239] N 2 -(1-cyclohexyl-1H-pyrazol-4-yl)-N 4 - Preparation of isopropyl-5-(1-methyl-1H-pyrazol-4-yl)-5H-pyrrolo[3,2-d]pyrimidine-2,4-diamine:
[0240]
[0241] The first step: the preparation of 2,4-dichloro-5-(1-methyl-1H-pyrazol-4-yl)-5H-pyrrole[3,2-d]pyrimidine
[0242]
[0243] 2,4-dichloro-5H-pyrrole[3,2-d]pyrimidine (376mg, 2mmol), 1-methyl-4-(4,4,5,5-tetramethyl-1,3,2 -Dioxaborolan-2-yl)-1H-pyrazole (832 mg, 4 mmol), anhydrous copper acetate (363 mg, 2 mmol) and pyridine (158 mg, 2 mmol) were added to dimethylsulfoxide (10 mL) . The reaction solution was heated to 110°C and stirred overnight. The reaction solution was naturally cooled to room temperature, added ethyl acetate (50mL), filtered to remove insoluble matter, the filtrate was washed with water (40mL×2), dried over anhydrous sodium sulfate, concentrated under reduced pressure, and washed with n-hexane / ethyl acetate (2 / 1 , 30mL) of the mixed solution was beaten and filtered to obtain 150mg of light ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com