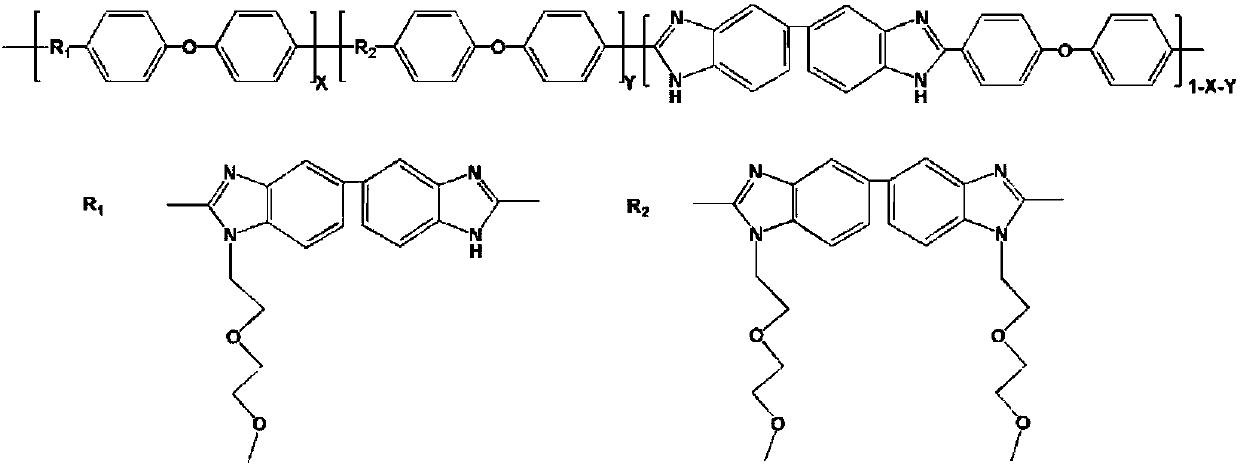
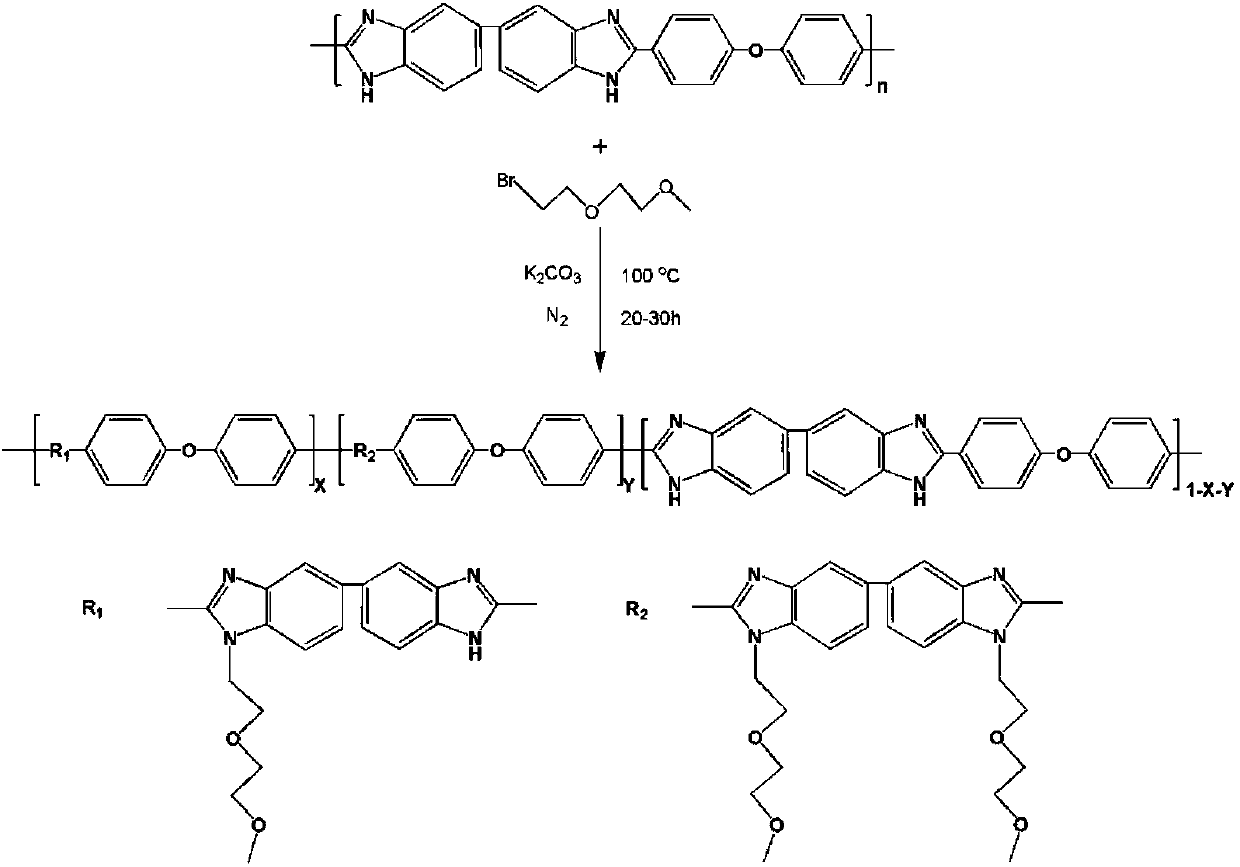
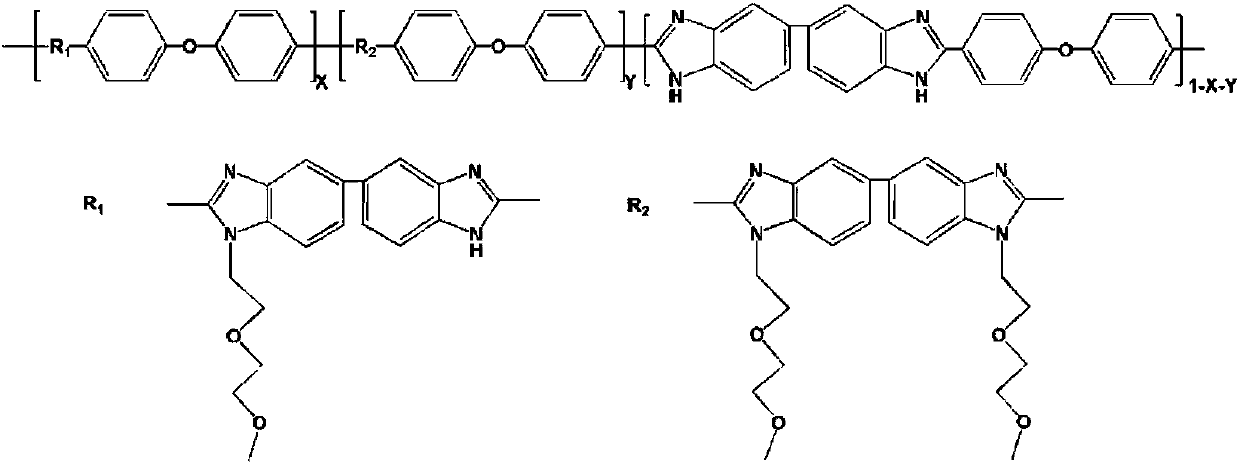
Nonionic hydrophilic side-chain polybenzimidazole membrane and preparation method thereof
A polybenzimidazole membrane, non-ionic technology, applied in the field of ion exchange membranes, can solve the problems of increasing the complexity of the membrane preparation process, achieve good chemical stability, good comprehensive performance, and improve the effect of proton conductivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Add 1 g of PBI powder and 33 g of dimethyl sulfoxide into a dry 100 ml three-necked flask, and heat to dissolve at 80°C. After the dissolution is completed, the temperature is raised to 100°C. Because the deprotonation and nucleophilic substitution reaction of polybenzimidazole is carried out at a high temperature of 100°C, an oil bath is used for heat preservation, and a serpentine condensation reflux device is installed on the reaction three-necked flask. Condensate and reflux in an ice-water bath. Under nitrogen protection, 345.5 mg of potassium carbonate powder was added, and the reaction was stirred for 2 hours. 686.4 mg of 1-bromo-2-(2-methoxyethoxy)ethane was mixed with 3 g of dimethyl sulfoxide, slowly dropped into the reaction solution, and stirred for 20 hours. After the reaction, the reaction device was cooled in the air. After the reaction solution was cooled to room temperature, it was slowly poured into dilute sulfuric acid with a concentration of about 0...
Embodiment 2
[0030] Add 27g of dimethyl sulfoxide into a dry 100ml three-neck flask, add 1g of PBI powder under stirring, heat and dissolve at 80°C to form a yellow transparent solution. After the dissolution is completed, the temperature is raised to 100° C., and an oil bath is used for heat preservation. A serpentine condenser is installed on the reaction three-necked flask, and ice water is used to condense and reflux. Nitrogen was purged in the reaction system, 691.0 mg of potassium carbonate powder was added, and the reaction was stirred for 2 hours. Then, 1.373 g of small hydrophilic molecules for grafting and 3 g of dimethyl sulfoxide were uniformly mixed, slowly dropped into the reaction solution, and the stirring reaction was continued for 20 hours. After the reaction, the three-neck flask was cooled in the air and kept under nitrogen purging. After the reaction liquid was cooled to room temperature, it was slowly poured into dilute sulfuric acid with a concentration of about 0.6 ...
Embodiment 3
[0034]Take a dry and clean 100ml three-neck flask, add 1g of PBI powder and 24g of dimethyl sulfoxide, stir and heat at 80°C to dissolve, forming a uniform and transparent yellow solution. Then the temperature was raised to 100° C., and an oil bath was used for heat preservation, and a serpentine condensing reflux device was installed on the reaction three-necked flask, and ice water was used for condensing and refluxing. In order to prevent the deprotonated PBI from being oxidized by oxygen in the air, the whole reaction was carried out under the protection of nitrogen. 1.036g of potassium carbonate powder was added, and the reaction was stirred for 2 hours. Mix 1.373g of small hydrophilic molecules for grafting with 4g of dimethyl sulfoxide, slowly drop them into the reaction solution, and continue to stir and react for 30 hours. After the reaction, the reaction device was cooled in the air. After the reaction solution was cooled to room temperature, it was slowly poured in...
PUM
| Property | Measurement | Unit |
|---|---|---|
| voltage efficiency | aaaaa | aaaaa |
| voltage efficiency | aaaaa | aaaaa |
| voltage efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com