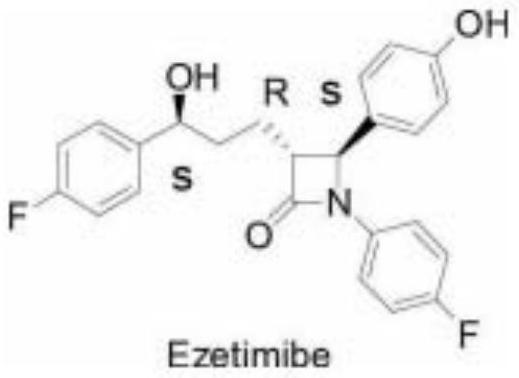
A kind of detection method of ezetimibe intermediate
A detection method, the technology of ezetimibe, applied in the field of analytical chemistry, can solve the problems affecting the curative effect of drugs, affecting the chiral purity of ezetimibe isomer impurity content, etc., to achieve accurate and reliable calculation results, reliable detection methods, fast response effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] A detection method for (4S)-3-[5-(4-fluorophenyl)-1,5-dioxopentyl]-4-phenyl-2-oxazolidinone,
[0039] 1. Instruments and testing conditions
[0040] Shimadzu LC-15C high performance liquid chromatography, the chromatographic column is chiralcel OD-H, its size is 4.6*250mm, 5μm;
[0041] Injection volume: 20μl;
[0042] Flow rate: 1ml / min;
[0043] Column temperature: 25°C;
[0044] Detection wavelength: 214nm;
[0045] Mobile phase: normal hexane: Virahol, its volume ratio is 700: 300;
[0046] Diluent: isopropanol;
[0047] Detector: UV detector.
[0048] 2. Experimental steps
[0049] Adopt high-performance liquid chromatography to detect, and the detection steps are as follows:
[0050] (1) Accurately weigh 25 mg of (4S)-3-[5-(4-fluorophenyl)-1,5-dioxopentyl]-4-phenyl-2-oxazolidinone and 25 mg (4R)-3-[5-(4-fluorophenyl)-1,5-dioxopentyl]-4-phenyl-2-oxazolidinone is placed in the same 100ml volumetric flask, Dissolve and dilute with diluent to prepare a syste...
Embodiment 2
[0055] Take three batches of (4S)-3-[5-(4-fluorophenyl)-1,5-dioxopentyl]-4-phenyl-2-oxazolidinone produced according to the same production specifications, Test products 1-3 were tested according to the detection method of Example 1, and the area normalization method was used to calculate the purity and corresponding isomers. The test results are shown in the following table 1:
[0056] Table 1 is the detection result of test product 1-3
[0057]
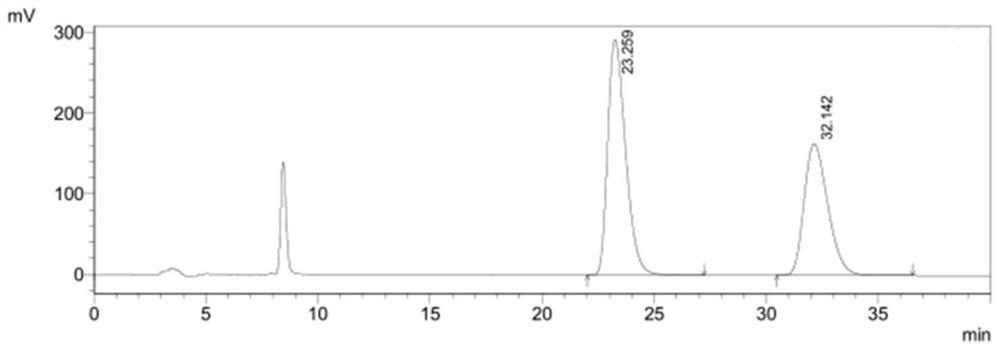
[0058] figure 1It is the high performance liquid chromatogram of the system adaptability solution in embodiment 1, and the peak order is (4S)-3-[5-(4-fluorophenyl)-1,5-dioxopentyl]-4- Phenyl-2-oxazolidinone, (4R)-3-[5-(4-fluorophenyl)-1,5-dioxopentyl]-4-phenyl-2-oxazolidinone.
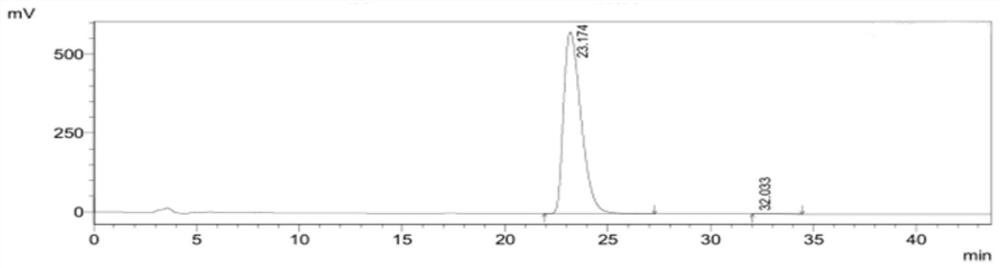
[0059] figure 2 It is the high performance liquid chromatogram of need testing product 1, and its detection result is shown in the following table 2. Wherein the peak at 23.174min is (4S)-3-[5-(4-fluorophenyl)-1,5-dioxopentyl]-4-phenyl-2-oxazolidinon...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com