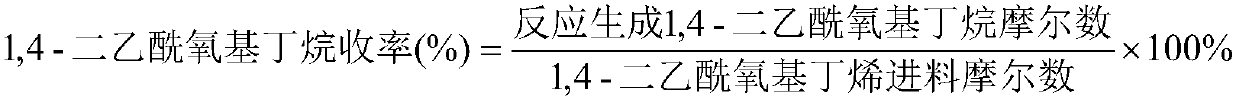Method for synthesizing 1,4-butanediol
A technology of butanediol and diacetoxybutene, applied in chemical instruments and methods, preparation of organic compounds, preparation of oxygenated compounds, etc., can solve the problems of low yield and selectivity of 4-butanediol
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Preparation of hydrogenation catalyst:
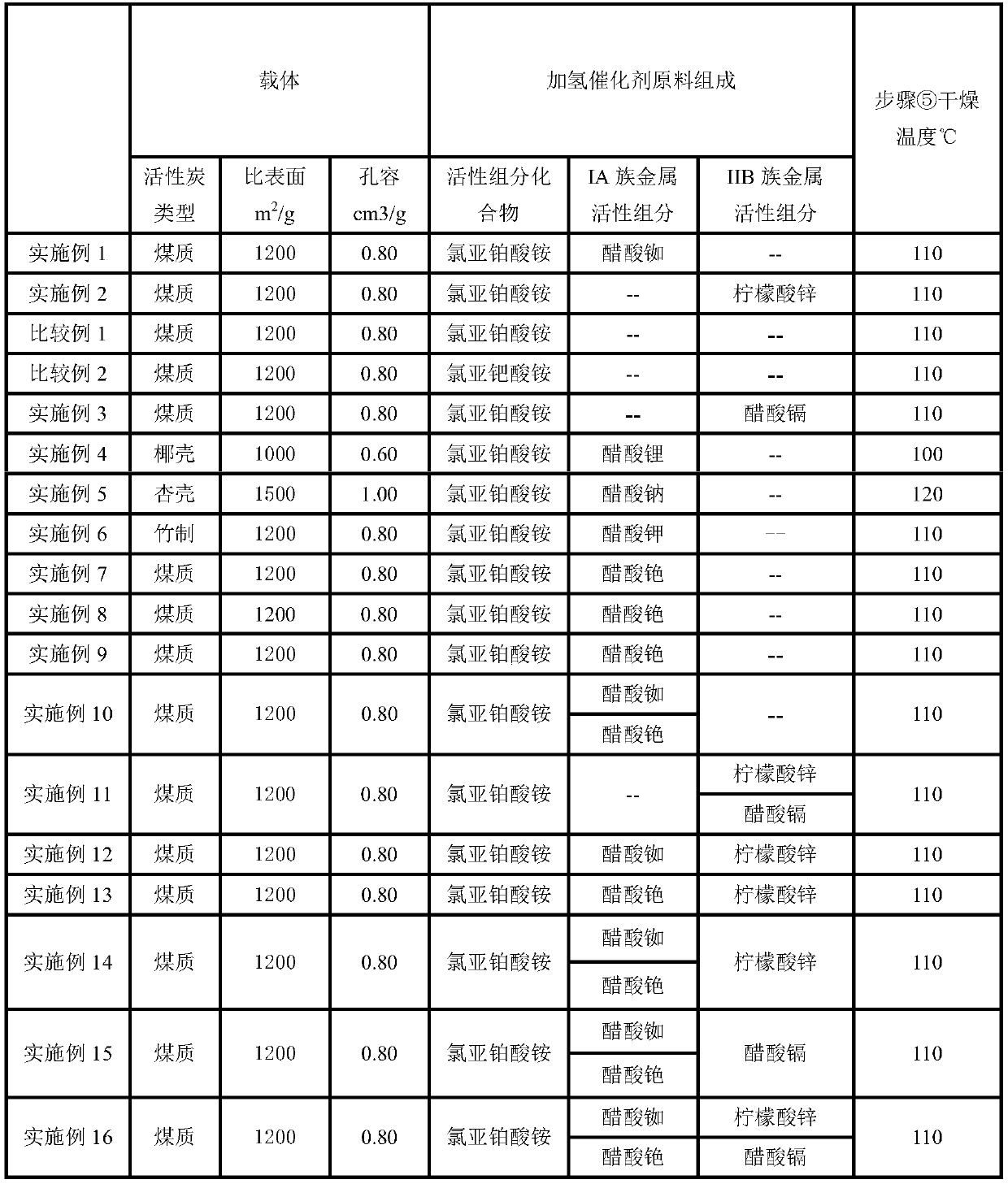
[0044] ① Ammonium chloroplatinite ((NH 4 ) 2 PtCl 4 ) is dissolved in the hydrochloric acid aqueous solution that concentration is 8wt%, obtains impregnation solution 200ml, and 1L diameter 3mm, length 2cm, pore volume are 0.80cm 3 / g, the specific surface area is 1200cm 2 The coal-based cylindrical activated carbon carrier of / g is impregnated in the above-mentioned impregnating liquid, obtains catalyst precursor I;
[0045] ② Standing and aging for 24 hours to obtain catalyst precursor II;
[0046] ③ with a concentration of 8% (in N 2 h 4 ·H 2 The 500ml hydrazine hydrate of (2 weight ratio) reduces catalyst precursor II for 3h, obtains catalyst precursor III;
[0047] ④ washed with water to no chloride ion, dried at 50°C for 4 hours to obtain catalyst precursor IV;
[0048] ⑤ impregnating 180 ml of an aqueous solution of rubidium acetate (RbOAc) containing 2.10 g of Rb on the catalyst precursor IV, and drying at 110° C...
Embodiment 2
[0054] Preparation of hydrogenation catalyst:
[0055] ① Ammonium chloroplatinite ((NH 4 ) 2 PtCl 4 ) is dissolved in the hydrochloric acid aqueous solution that concentration is 8wt%, obtains impregnation solution 200ml, and 1L diameter 3mm, length 2cm, pore volume are 0.80cm 3 / g, the specific surface area is 1200cm 2 The coal-based cylindrical activated carbon carrier of / g is impregnated in the above-mentioned impregnating liquid, obtains catalyst precursor I;
[0056] ② Standing and aging for 24 hours to obtain catalyst precursor II;
[0057] ③ with a concentration of 8% (in N 2 h 4 ·H 2 The 500ml hydrazine hydrate of (2 weight ratio) reduces catalyst precursor II for 3h, obtains catalyst precursor III;
[0058] ④ washed with water to no chloride ion, dried at 50°C for 4 hours to obtain catalyst precursor IV;
[0059] ⑤ Zinc citrate containing 2.10g Zn (Zn 3 (C 6 h 5 o 7 ) 2 2H 2 O) 180ml of aqueous solution was impregnated on the catalyst precursor IV, and...
Embodiment 3
[0089] Preparation of hydrogenation catalyst:
[0090] ① Ammonium chloroplatinite ((NH 4 ) 2 PtCl 4 ) is dissolved in the hydrochloric acid aqueous solution that concentration is 8wt%, obtains impregnation solution 200ml, and 1L diameter 3mm, length 2cm, pore volume are 0.80cm 3 / g, the specific surface area is 1200cm 2 The coal-based cylindrical activated carbon carrier of / g is impregnated in the above-mentioned impregnating liquid, obtains catalyst precursor I;
[0091] ② Standing and aging for 24 hours to obtain catalyst precursor II;
[0092] ③ with a concentration of 8% (in N 2 h 4 ·H 2 The 500ml hydrazine hydrate of (2 weight ratio) reduces catalyst precursor II for 3h, obtains catalyst precursor III;
[0093] ④ washed with water to no chloride ion, dried at 50°C for 4 hours to obtain catalyst precursor IV;
[0094] ⑤The cadmium acetate containing 2.10g Cd (Cd(OA C ) 2 2H 2 O) Dissolved in an aqueous solution of 10 wt% acetic acid to obtain 180 ml of impregn...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com