Application of inactivated lactic acid bacteria in medicine for preventing and controlling vertical transmission diseases of sow
A technology of inactivated lactic acid bacteria and vertical transmission, which is applied in the new application field of inactivated lactic acid bacteria in the prevention and control of vertically transmitted diseases of sows, so as to improve the survival rate and health level of piglets and reduce the incidence of disease
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Inoculate Enterococcus faecium (purchased from China Industrial Microorganism Culture Collection and Management Center, Latin name: Enterococcusfaecium, preservation number: CICC 6049) in MRS medium, cultivate in an incubator at 37°C for 24 hours, then centrifuge at 3000 rpm for 5 minutes, remove Keep the precipitate in the upper layer culture solution, add sterile normal saline to wash the precipitate, centrifuge for 5 minutes, repeat the washing 3 times, add sterile normal saline, and mix with the precipitate. Get a certain amount of Enterococcus faecium physiological saline suspension, measure its OD value at 690nm place of the spectrophotometer, when the OD value of the final concentration diluted with sterile physiological saline is 0.38, the Enterococcus faecium physiological The saline suspension is used as a 1-fold (1×) concentration, and the bacteria counted by the THOMA bacterial counting plate can be determined to contain about 10 per mL of the suspension at t...
Embodiment 2
[0017] The suspension prepared in Example 1 was diluted to prepare a suspension of inactivated Enterococcus faecium at a concentration of 10×, and the effect of intravenous injection of inactivated lactic acid bacteria on vertically transmitted diseases in sows was detected. Respiratory tract syndrome and multi-system failure syndrome occurred in piglets previously weaned in a pig farm, mainly caused by the vertical transmission of porcine reproductive and respiratory syndrome virus and porcine circovirus. The rate is above 50%. In March 2016, 5 mL of inactivated Enterococcus faecium suspension at a concentration of 10× was injected into the ear vein of 8 sows on the day of farrowing and the next day respectively. Thereafter, observe and record the incidence of piglets after weaning. A total of 76 piglets were produced by 8 sows, only 4 of which developed respiratory syndrome and multi-system failure syndrome after weaning, the incidence rate was 5.3%. Compared with before, ...
Embodiment 3
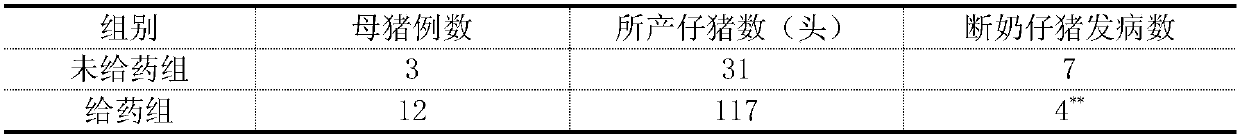
[0019] Plant Lactobacillus plantarum subspecies (purchased from China Industrial Microorganism Culture Collection Management Center, Latin name: Lactobacillus plantarum subsp.Plantarum, preservation number: CICC 6240) was prepared according to the method of Example 1. Inactivated Lactobacillus plantarum at a concentration of 10× Subsp. planta suspension, to detect the effect of inactivated Lactobacillus plantarum subsp. planta suspension on vertically transmitted diseases in sows. In a pig farm, 10 mL of inactivated Lactobacillus plantarum subsp. plantarum suspension was injected into the ear veins of 12 sows on the day of delivery and the next day, and 3 sows were not administered as a control. Observe and record the incidence of weaning piglets. A total of 117 piglets were delivered by intravenous injection of inactivated Lactobacillus plantarum subsp. Caused by vertical transmission of rabies virus, the incidence rate was 4 / 117 (3.4%), and no one died. Among the 31 weaned...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com