Arenobufagin derivative as well as preparation method and application thereof
A technology of sandbuad venom and its derivatives, which is applied in the field of medicine and can solve the problems of enhancing the toxicity of compounds and high toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
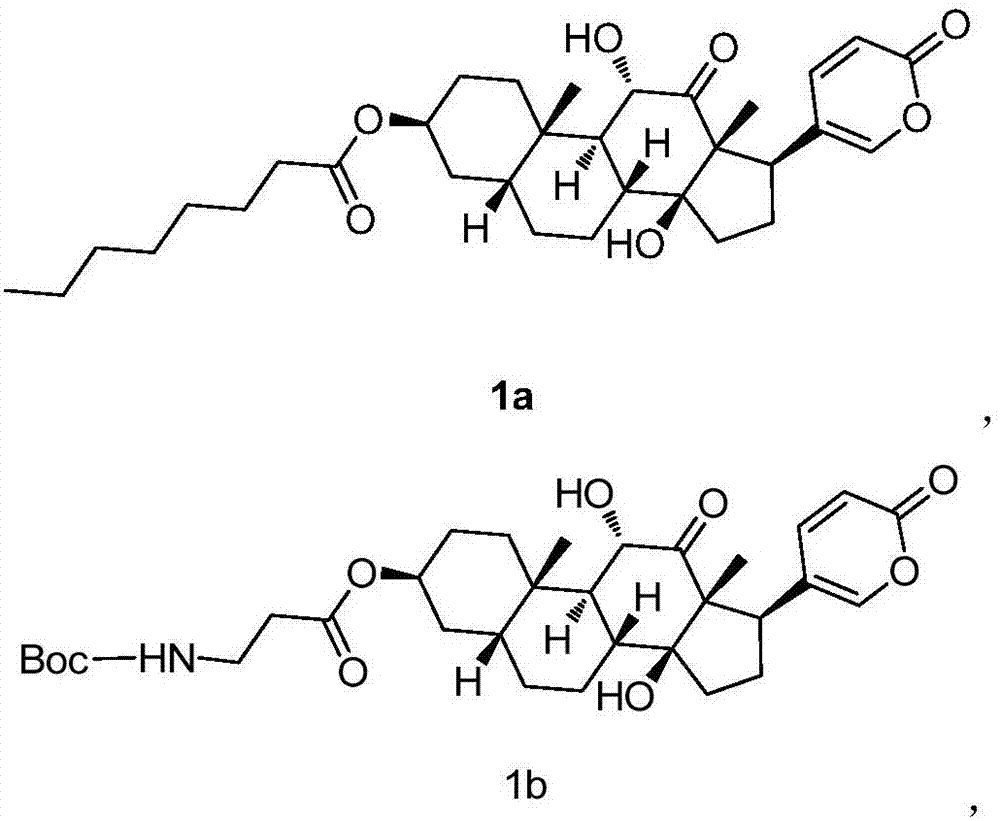
[0063] Embodiment 1, the preparation of compound 1a
[0064]
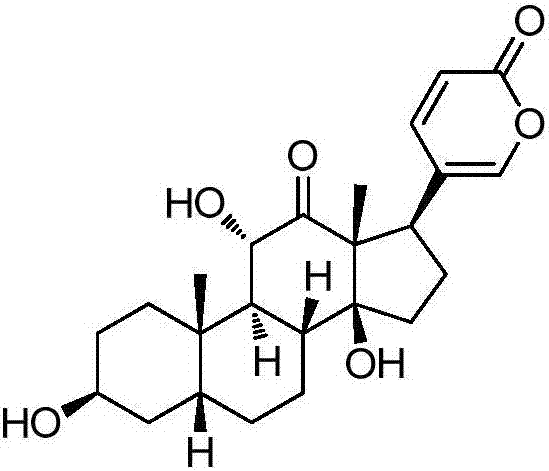
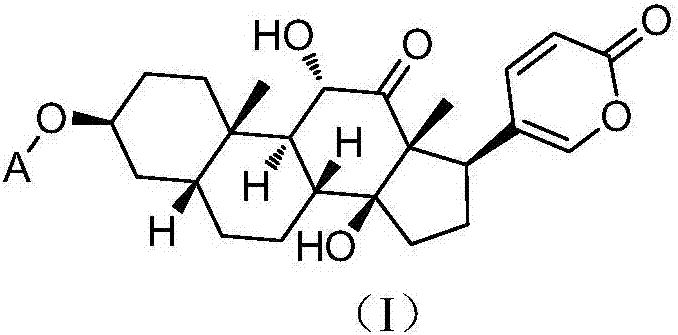
[0065] Dissolve sabubaptin (83mg, 0.2mmol) in 10mL of dry dichloromethane, add triethylamine (81mg, 0.8mmol), and then add n-octanoyl chloride (129.6mg, 0.8mmol) dropwise. After the dropwise addition, Stir overnight at room temperature. Purified by column chromatography to obtain 60 mg of white solid 1a with a yield of 55.5%. 1 H NMR: (CDCl 3 ,500MHz) δ: 7.76(d,1H),7.41(s,1H),6.27(d,1H),5.3(s,1H),4.33(d,1H),4.11(d,1H),3.84(s ,1H),2.43(d,1H),2.30(t,2H),2.05-2.09(m,2H),1.86-1.94(m,2H),1.75-1.80(m,5H),1.63-1.73(m ,4H), 1.45(d,1H), 1.33-1.44(m,15H), 1.27(s,3H), 0.86-0.88(m,3H).
Embodiment 2
[0066] Embodiment 2, the preparation of compound 1b
[0067]
[0068] Dissolve sabubaptin (83 mg, 0.2 mmol) in 10 mL of dry dichloromethane, add DMAP (4-dimethylaminopyridine) (24.4 mg, 0.2 mmol), Boc-alanine (tert-butoxycarbonyl -L-alanine) (56.8mg, 0.3mmol), then add EDCI (1-(3-dimethylaminopropyl)-3-ethylcarbodiimide) (115mg, 0.6mmol), stir at room temperature overnight . Purified by column chromatography to obtain 50 mg of white solid 1b with a yield of 43.2%. 1 H NMR: (CDCl 3,500MHz) δ: 7.76(d,1H),7.41(s,1H),6.27(d,1H),5.13(s,1H),5.04(s,1H),4.34-4.36(m,1H),4.12 -4.16(m,1H),3.85(d,1H),3.42(d,2H),2.54-2.57(m,2H),2.46(d,1H),2.04-2.08(m,3H),1.96-2.00 (m,1H),1.65-1.85(m,7H),1.57-1.62(m,4H),1.40-1.46(m,9H),1.19-1.23(m,3H),0.92-0.96(m,3H) .
Embodiment 3
[0069] Embodiment 3, the preparation of compound 1c
[0070]
[0071] Sabubuadin (83mg, 0.2mmol) was dissolved in 10mL of dry dichloromethane, DMAP (24.4mg, 0.2mmol), Boc-phenylalanine (N-tert-butoxycarbonyl-L- Phenylalanine ) (79.6mg, 0.3mmol), then added EDCI (115mg, 0.6mmol), and stirred at room temperature overnight. Purified by column chromatography to obtain 52 mg of white solid 1c with a yield of 40.2%. 1 H NMR: (CDCl 3 ,500MHz)δ: 7.76(d,1H),7.41(s,1H),7.23-7.31(m,4H),7.15-7.16(m,2H),6.27(d,1H),5.07-5.12(m, 2H),4.58(d,1H),4.31-4.34(m,1H),4.09-4.12(m,1H),3.83-3.86(m,1H),3.06-3.10(m,2H),2.06(s, 1H),2.38(d,1H),2.06-2.08(m,2H),1.82-1.89(m,3H),1.67-1.79(m,6H),1.65-1.66(m,1H),1.31-1.37( m,5H), 1.21-1.23(m,10H), 0.91-0.97(m,3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com