System for determining individual effectiveness of tripterygium glycosides tablets for treating rheumatoid arthritis by expression quantity of plurality of mRNAs
A rheumatoid, expression-level technology, applied in the determination/inspection of microorganisms, bioreactors/fermenters for specific purposes, biochemical instruments, etc. Toxic dose equivalency issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] This example is used to illustrate the discovery of biomarkers and the establishment of prediction models of the present disclosure.
[0043] Source of cases and sample size: 40 RA patients who met the inclusion criteria in the rheumatology department and outpatient department of Guang'anmen Hospital, China Academy of Chinese Medical Sciences. At the same time, 10 healthy volunteers from the Health Examination Center were selected, and the age and gender matched the disease group.
[0044] Inclusion criteria: in line with the American College of Rheumatology (American College of Rheumatology, ACR) classification criteria for RA in 1987 and the classification criteria for rheumatoid arthritis in 2010 (ACR / EULAR), never received tripterygium glycosides tablet treatment or in the last 4 days Subjects who have not received the treatment of tripterygium glycosides tablets within a week.
[0045] The classification criteria for rheumatoid arthritis revised by the American Co...
Embodiment 2
[0105] This example serves to illustrate the validation of the disclosed biomarkers and predictive models.
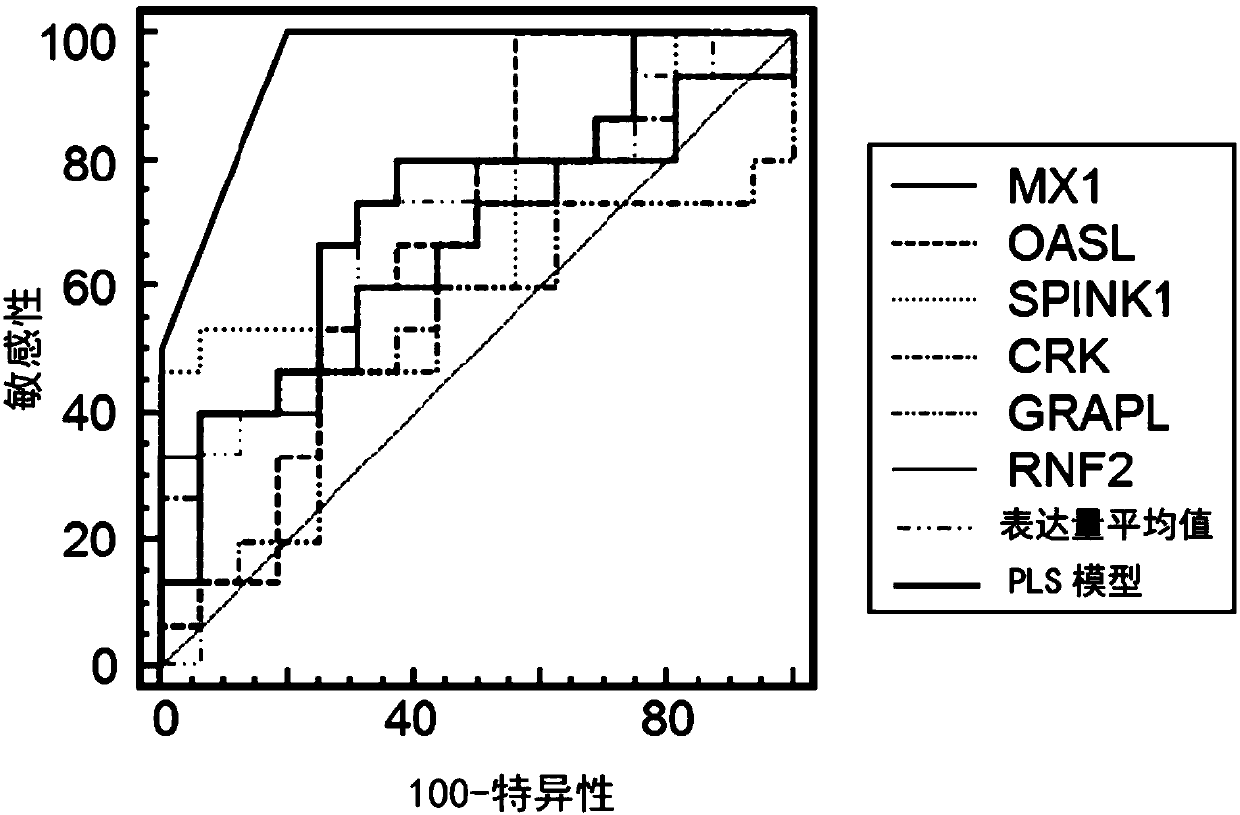
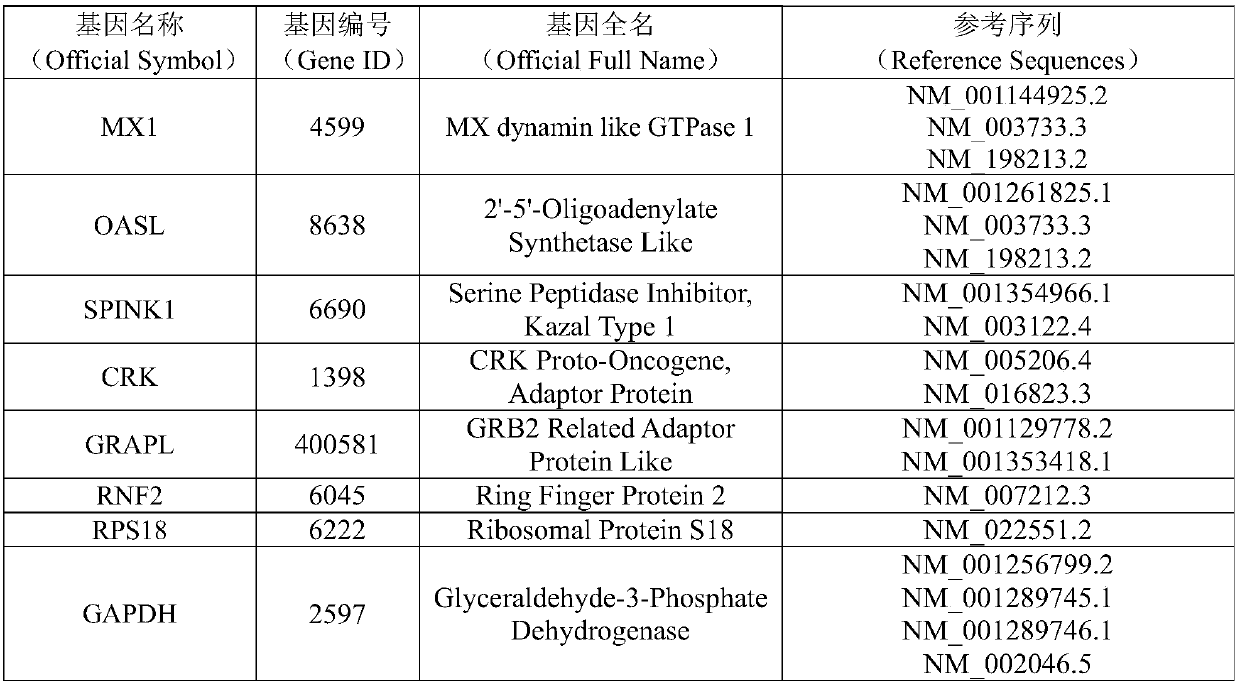
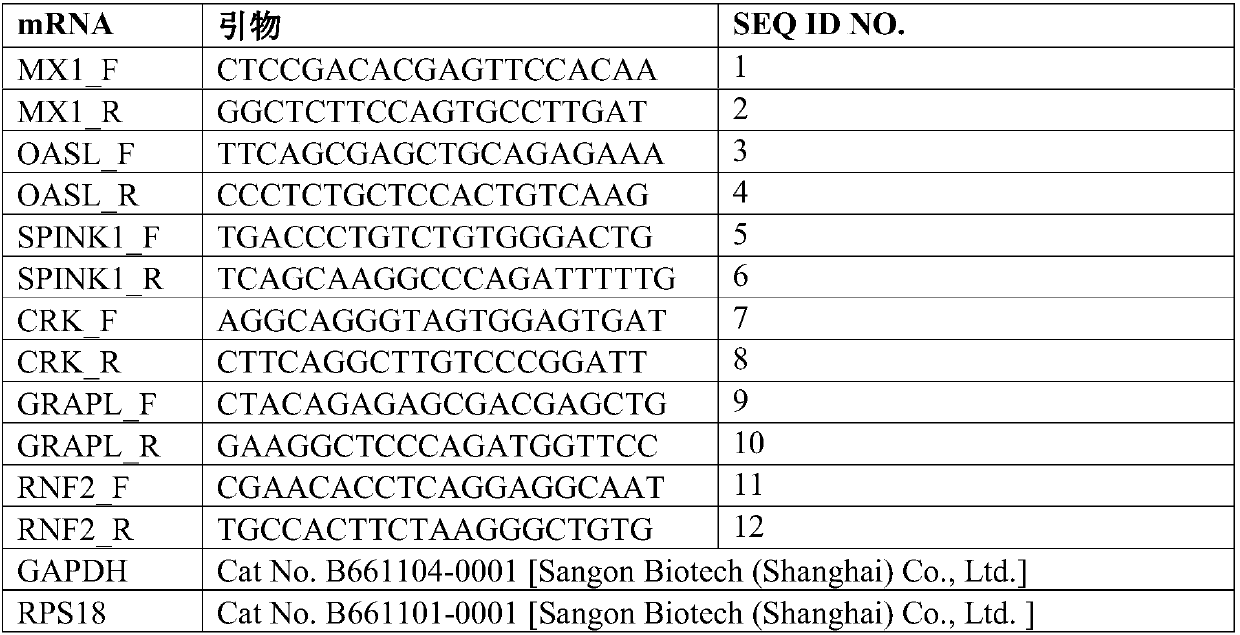
[0106] Reselect 60 cases of RA patients as an independent test set, and the inclusion and exclusion criteria are the same as in Example 1; taking Tripterygium wilfordii polyglycoside tablets for 12 weeks, during which regular follow-ups are performed to detect the indicators of standard treatment, and these RA patients are divided into three groups according to the clinical survey scale: Two groups: standard treatment group and non-standard treatment group; use real-time quantitative PCR (qRT-PCR) to detect the expression levels of MX1, OASL, SPINK1, CRK, GRAPL and RNF2 in the above independent test samples, and further verify the The performance of the curative effect prediction model for individualized treatment of RA, the evaluation indicators include prediction accuracy (accuracy, ACC) receiver operating characteristic curve (receiver operating characteristic curve, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com