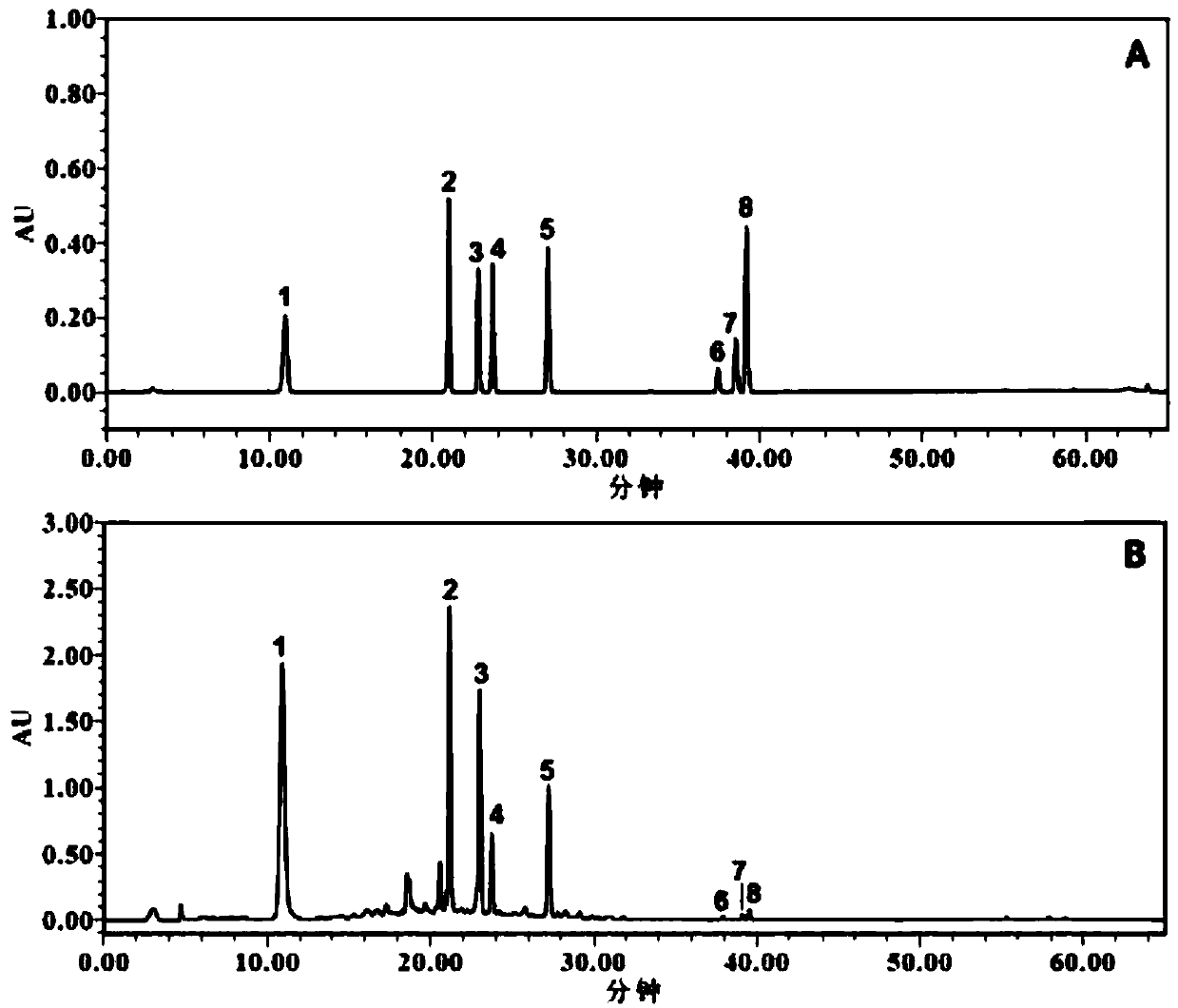
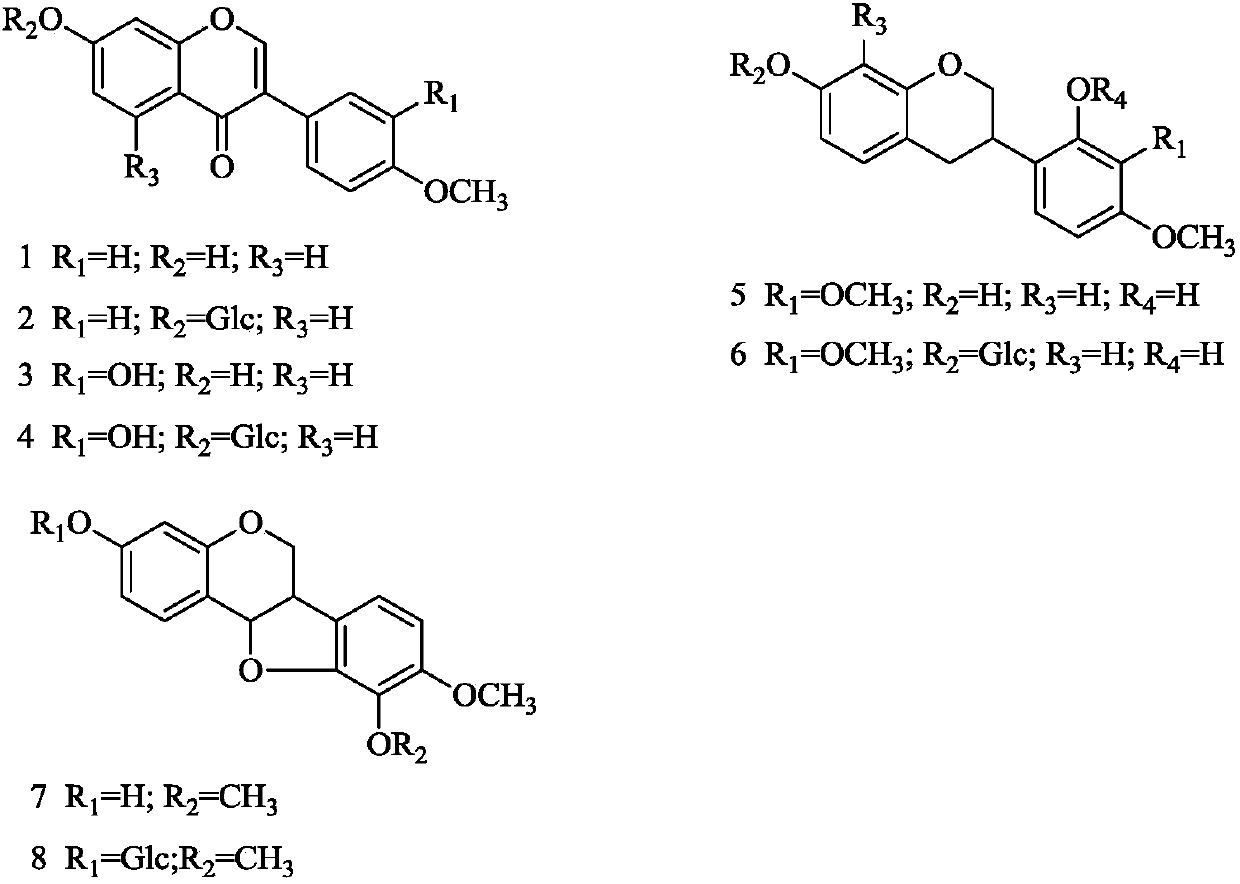
Application of astragalus flavonoid extract to preparation of drug for treating nephrotic syndrome
A technology for nephrotic syndrome and astragalus flavonoids is applied in the directions of drug combination, pharmaceutical formula, plant raw materials, etc., can solve the problems of unclear active ingredients, limited efficacy and the like, and achieves low cost, lower urine protein level, and simple extraction process. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] The preparation of embodiment 1 Astragalus flavonoids extract
[0017] Take 3000g of Radix Astragali, crush it, add 8L of water, heat and reflux for extraction 3 times, each time for 1 hour, combine the obtained extracts, concentrate under reduced pressure to obtain a paste. Separate the above paste with AB-8 macroporous adsorption resin column, elute with water, 10%, and 35% ethanol in sequence, collect the 35% ethanol eluate, concentrate and dry under reduced pressure, and concentrate the above 35% ethanol eluate The product was dispersed in 200mL of water, extracted twice with 1 times the volume of ethyl acetate, the extracts were combined, concentrated under reduced pressure to obtain an extract, and dried in vacuo at 60°C to obtain 3375mg of astragalus flavonoids extract.
Embodiment 2
[0018] The preparation of embodiment 2 astragalus flavonoids extract
[0019] Take 1000 g of Radix Astragali, add 8 L of 20% ethanol, heat and reflux for extraction twice, each time for 2 hours, combine the obtained extracts, concentrate under reduced pressure to obtain a paste. Separate the above paste with AB-8 macroporous adsorption resin column, elute with water, 10%, and 35% ethanol in sequence, collect the 35% ethanol eluate, concentrate and dry under reduced pressure, and concentrate the above 35% ethanol eluate The product was dispersed in 200mL water, extracted once with 2 times the volume of ethyl acetate, the extracts were combined, concentrated under reduced pressure to obtain an extract, and dried in vacuo at 60°C to obtain 943 mg of astragalus flavonoids extract.
Embodiment 3
[0020] The preparation of embodiment 3 astragalus flavonoids extract
[0021] Take 1000 g of Radix Astragali, add 8 L of 95% ethanol, heat and reflux for extraction twice, each time for 1 hour, combine the obtained extracts, concentrate under reduced pressure to obtain a paste. Separate the above paste with AB-8 macroporous adsorption resin column, elute with water, 10%, and 35% ethanol in sequence, collect the 35% ethanol eluate, concentrate and dry under reduced pressure, and concentrate the above 35% ethanol eluate The product was dispersed in 200mL of water, extracted twice with 2 times the volume of ethyl acetate, the extracts were combined, concentrated under reduced pressure to obtain an extract, and dried in vacuo at 60°C to obtain 1064 mg of astragalus flavonoids extract.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com