Specific antibody targeting CD19, car-nk cell and its preparation and application
An antibody and cell technology, applied in the field of biomedicine, can solve the problems of inability to reinfuse the allogeneic body, the risk of large immune rejection, etc., and achieve the effect of stable traits
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] Example 1 Screening of CD19 Human Source Antibodies
[0065] 2 × YT liquid medium: 16 g of protein, 10 g yeast extract, 5 g sodium chloride, 800 ml of water, pH to 7.0 with sodium hydroxide, add water to 1000 mL, 20 min at 121 ° C.
[0066] 2 × YT-G: 2 × YT medium was added 2% glucose.
[0067] The 2 × YT-AK: 2 x YT medium was added 100 g / ml of ampicillin and 50 g / ml kanamycin.
[0068] First, prepare M13KO7 auxiliary phage
[0069] 1. Under 37 ° C, the long-term TG1 bacterial cells were infected with different dilution concentrations for 30 minutes, then coated it on the agar plate.
[0070] 2. TG1 bacteria collealed to 3 ml of liquid 2YT medium for culture. It was cultured for 2 hours at 37 ° C.
[0071] 3. Transfer the culture in step 2 to 1 L 2YT medium, and the carnicin to 50 μg / m, and incubation for 16 hours at 37 ° C.
[0072] 4. Centrifugation (10min AT 5000g) Remove bacterial cells, adding phage precipitates to the supernatant to collect phage.
[0073] 5. Ca...
Embodiment 2
[0088] Example 2: Expression and Identification of CD19 antibodies
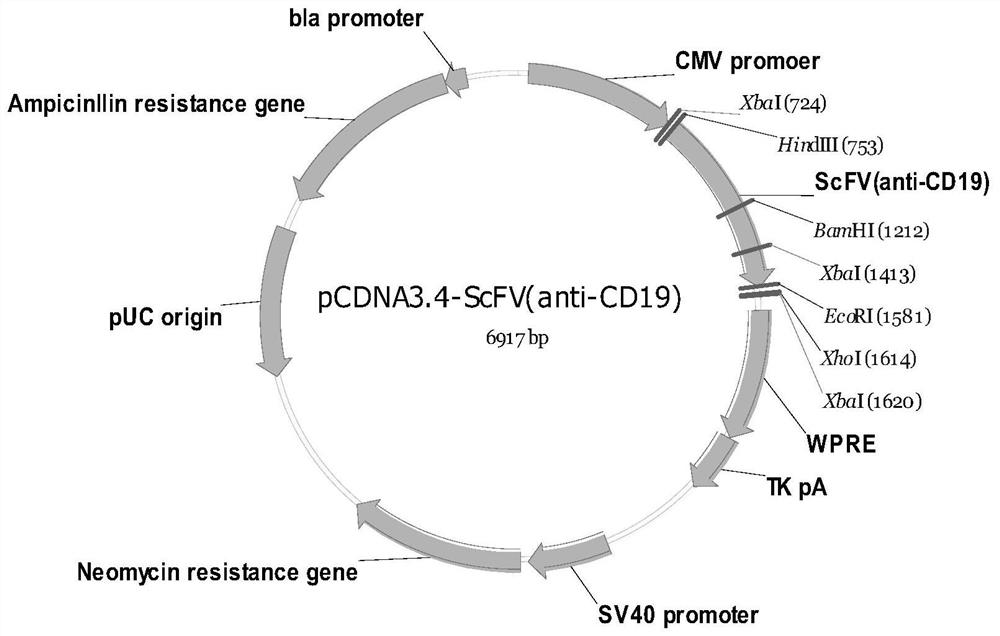
[0089] The single-stranded phage DNA obtained in Example 1 was cloned to the PCDNA3.4 vector, and constructed Figure 1 The PCDNA3.4-SCFV (Anti-CD19) expression vector was transiently expressed to CHO cells, purified by nickel strains for subsequent analysis.
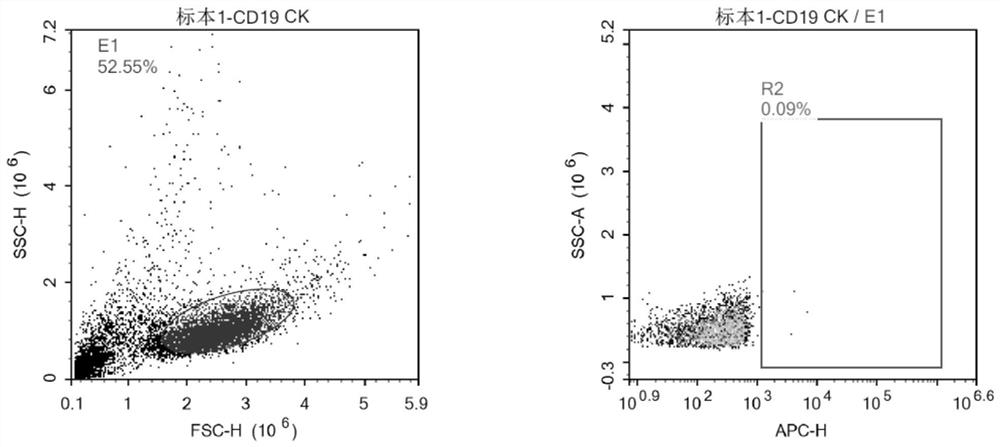
[0090] The fractional single-chain antibody to the CD19 protein was identified by the SPR method, and the highest affinity of the CD19 protein was selected from which the affinity of the CD19 protein was selected, and it was named a single chain antibody 2-27. Single-chain antibody 2-27 and CD19 molecular affinity are shown in Table 1.
[0091] Table 1: Single-chain antibody 2-27 and CD19 molecular affinity
[0092] Sequence KA (1 / ms) KD (1 / s) KD (M) 2-27 4.984E+4 0.004223 8.473E-8
[0093] Among them, the amino acid sequence of the VH chain of single-chain antibody 2-27 is shown in SEQ ID NO.7, and the nucleotide sequence is show...
Embodiment 3
[0094] Example 3 Preparation of a slow viral expression vector
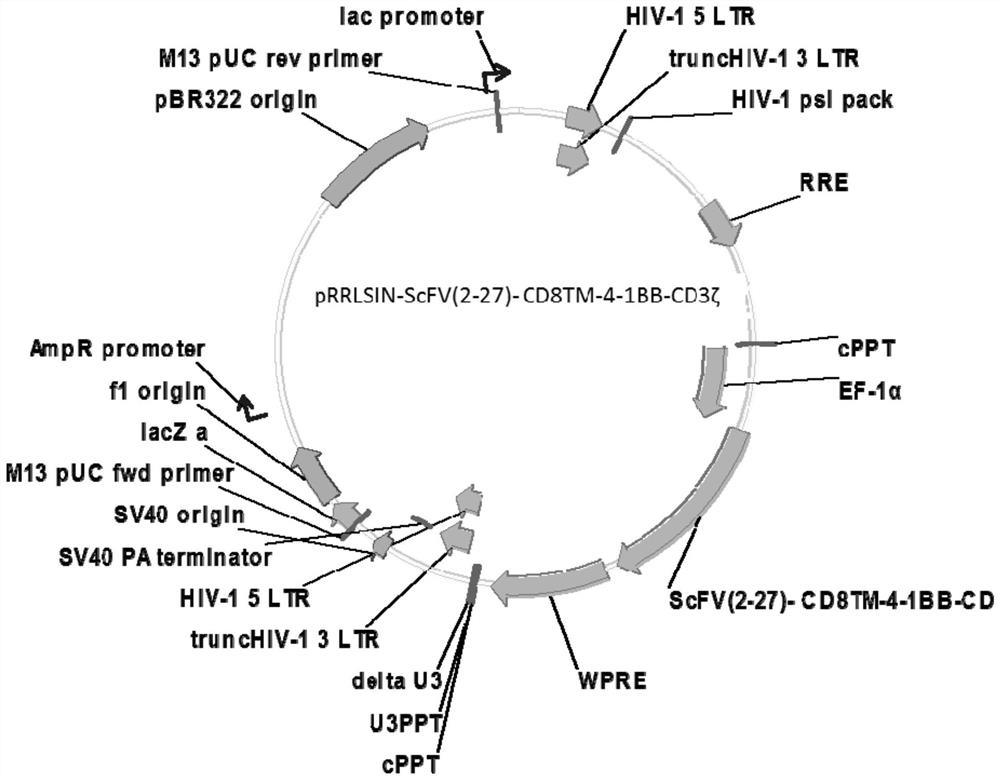
[0095] Gene Synthesis SCFV (2-27) -CD8-4-1BB-CD3ζ Fusion Gene sequence (which is shown in SEQ ID NO: 13, shown in SEQ ID NO: 14). Through the digestion, it is converted to the PRRSL IN vector, and the gene is upstream of the EP-1α promoter. The vector transformed STBL3 Escherichia coli strain, ampicillin screening, gaining positive clones, extracting plasmid, and cloning, obtained Prrlsin-SCFV (2-27) -CD8-4-1BB-CD3ζ slow virus transfection carrier (eg figure 2 Indicated).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com